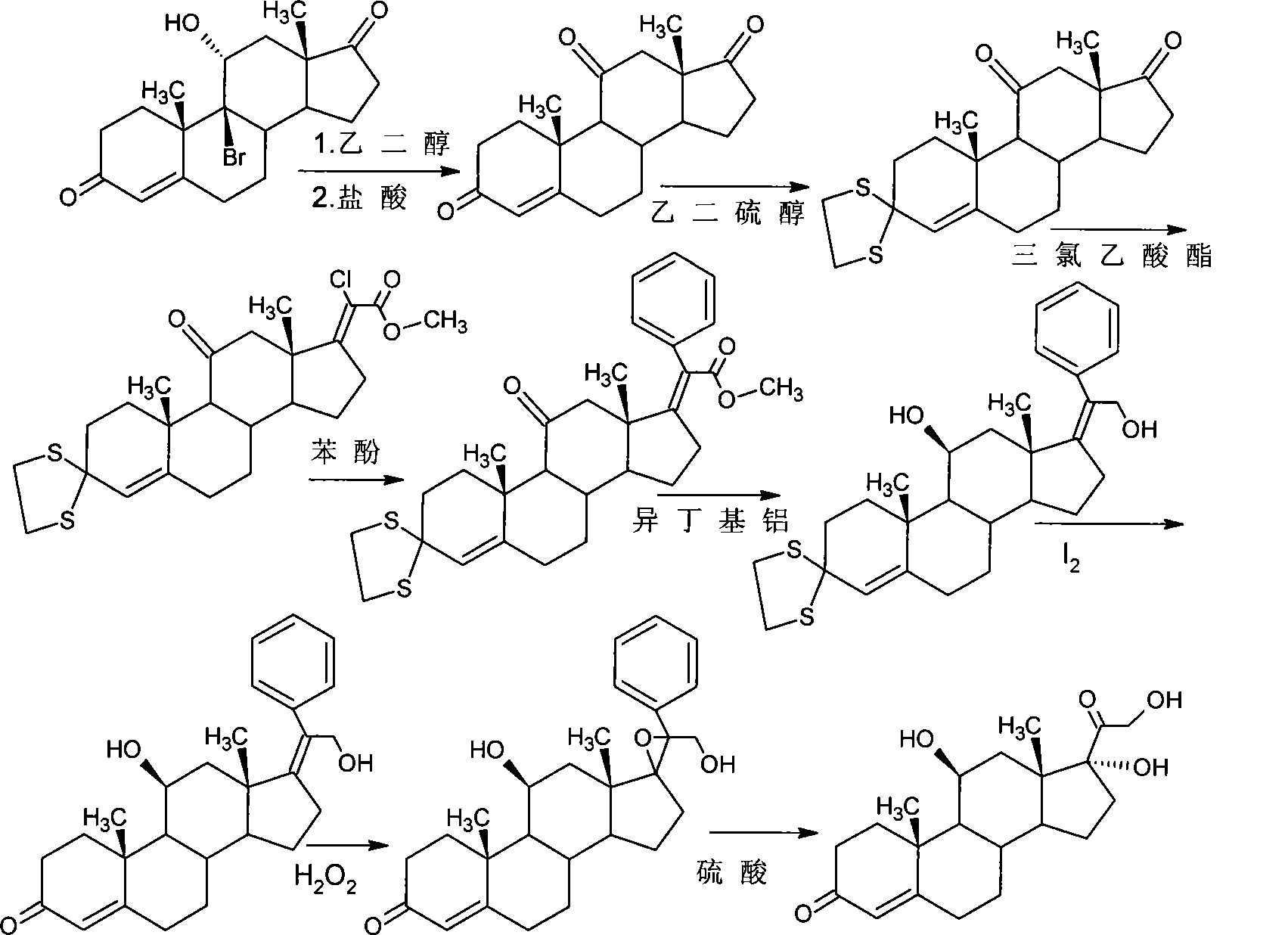
Preparation of hydrocortisone
A technology for hydrocortisone and a compound, which is applied to a preparation field of steroid compounds, can solve the problems of low degree of industrialization, high cost, long route and the like, and achieves reduction of production cost and industrialization conditions, high feasibility and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
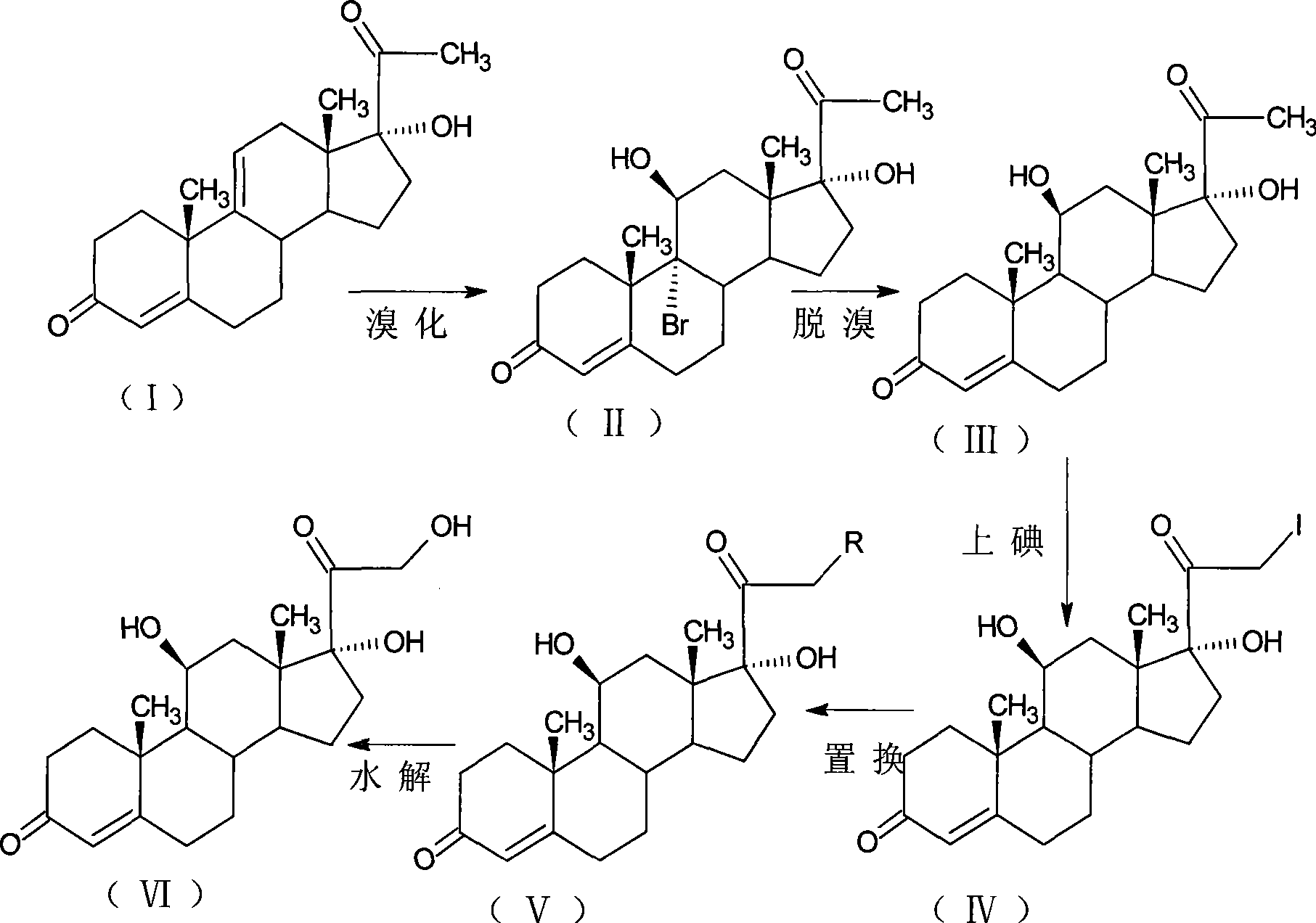
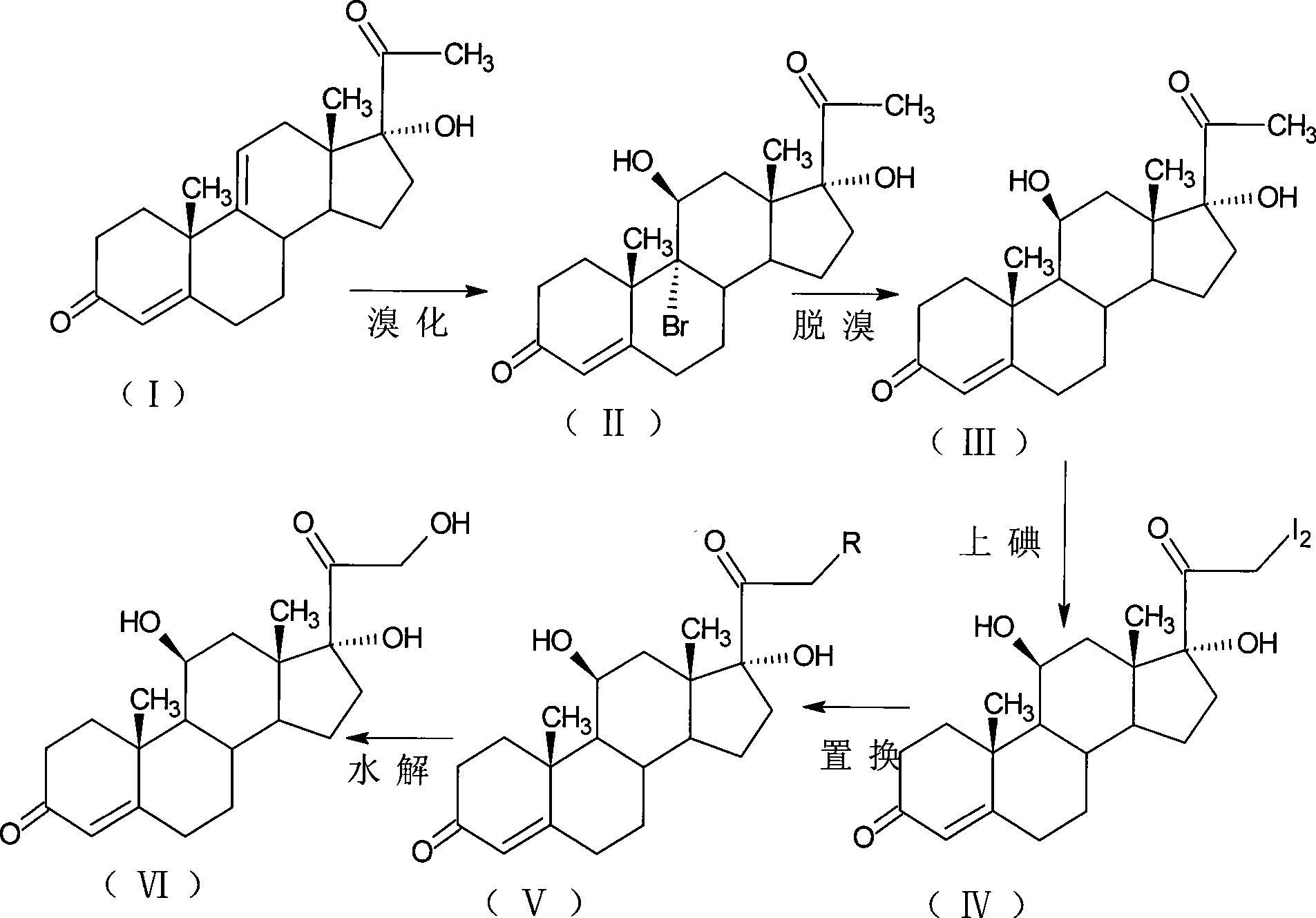
[0035] Bromination reaction: 9α-bromo-11β, 17α-dihydroxy-4-pregnene-3,20-dione;
[0036] Add 10g of 17-hydroxy-4,9-diene-pregna-3,20-dione (CN1896090) and 50ml of tetrahydrofuran into the reaction flask, stir, cool down to 0°C, and add dibromocyanide within 30 minutes Acetamide 6.5g, kept at 5-10°C for 1 hour, added 10% sodium bicarbonate aqueous solution to neutralize to PH=6.5, diluted in water, filtered, dried to obtain 11.5g of 9α-bromo-11β, 17α -Dihydroxy-4-pregnene-3,20-dione.
[0037] Debromination reaction: 11β, 17α-dihydroxy-4-pregnene-3,20-dione;
[0038] Add 11.5g of 9α-bromo-11β, 17α-dihydroxy-4-pregnene-3,20-dione, 100ml of DMF to the reaction flask, blow nitrogen, heat and control the temperature at 80-90°C, quickly drop 20ml of reducing agent for hydrogenation Tributyltin, after reacting for 1 hour, lower the temperature to 30°C, pour into 800ml saturated sodium chloride solution for dilution, stir for 1 hour, let stand for 1 hour, filter, wash with water unti...
Embodiment 2
[0046] Bromination reaction: 9α-bromo-11β, 17α-dihydroxy-4-pregnene-3,20-dione;
[0047] Add 10g of 17-hydroxy-4,9-diene-pregna-3,20-dione (CN1896090), 100ml of acetone into the reaction flask, stir, cool down to 0°C, and add N-bromine within 30 minutes 9g of substituted succinimide, kept at 5-10°C for 1 hour, added 10% aqueous sodium bicarbonate solution to neutralize to pH=6.5, diluted in water, filtered, and dried to obtain 11.2g of 9α-bromo-11β, 17α-Dihydroxy-4-pregnene-3,20-dione.
[0048] Debromination reaction: 11β, 17α-dihydroxy-4-pregnene-3,20-dione;
[0049] Prepare the reducing agent: take 10g of chromium particles in the reaction bottle, pass nitrogen, add 10ml of concentrated hydrochloric acid, react at room temperature for 30 minutes, and set aside.
[0050] In another reaction flask, add 12.5g of 9α-bromo-11β, 17α-dihydroxy-4-pregnene-3,20-dione, 60ml of dimethylformamide, blow nitrogen, cool down to 10°C, and slowly add The prepared chromium reducing agent w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com