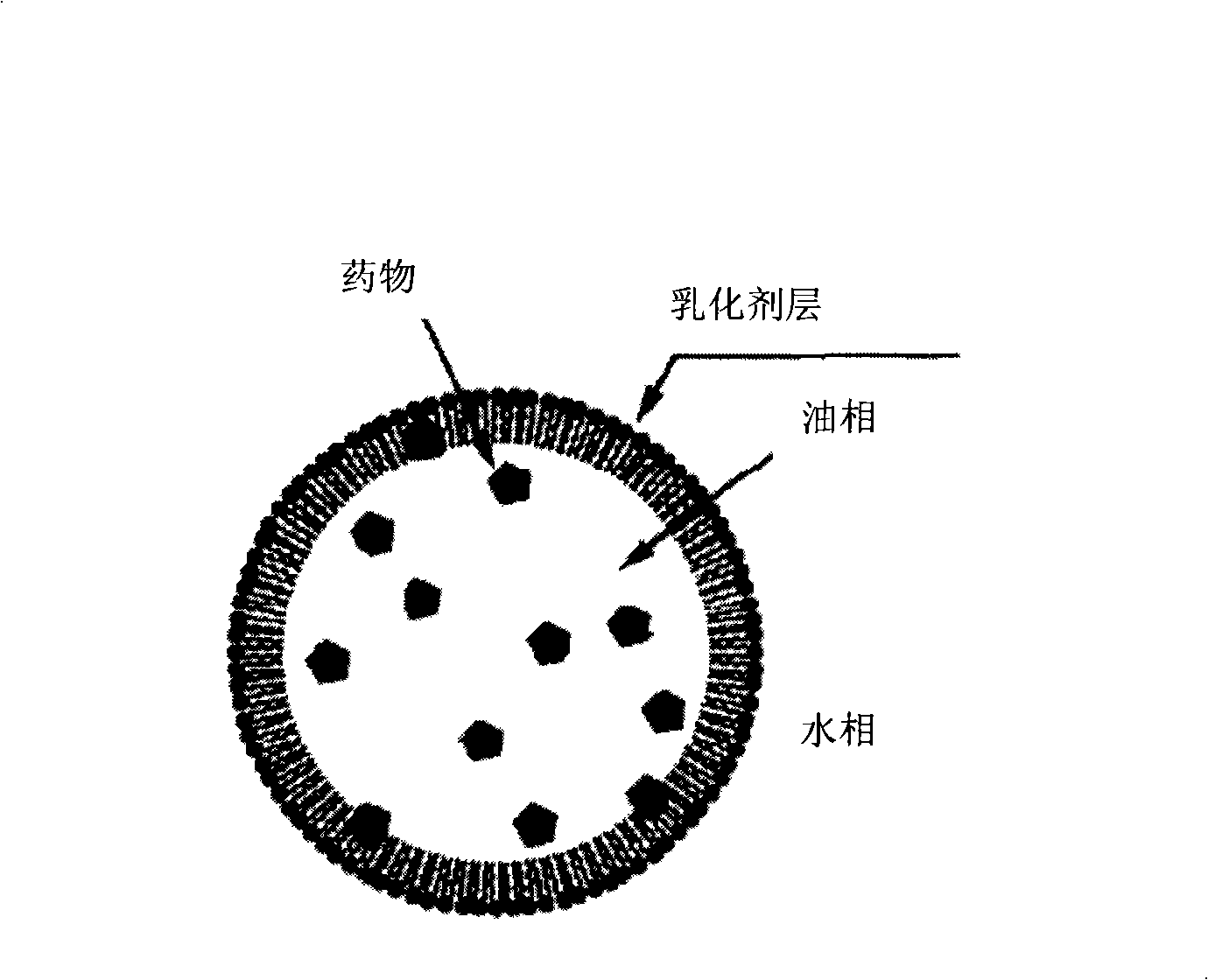
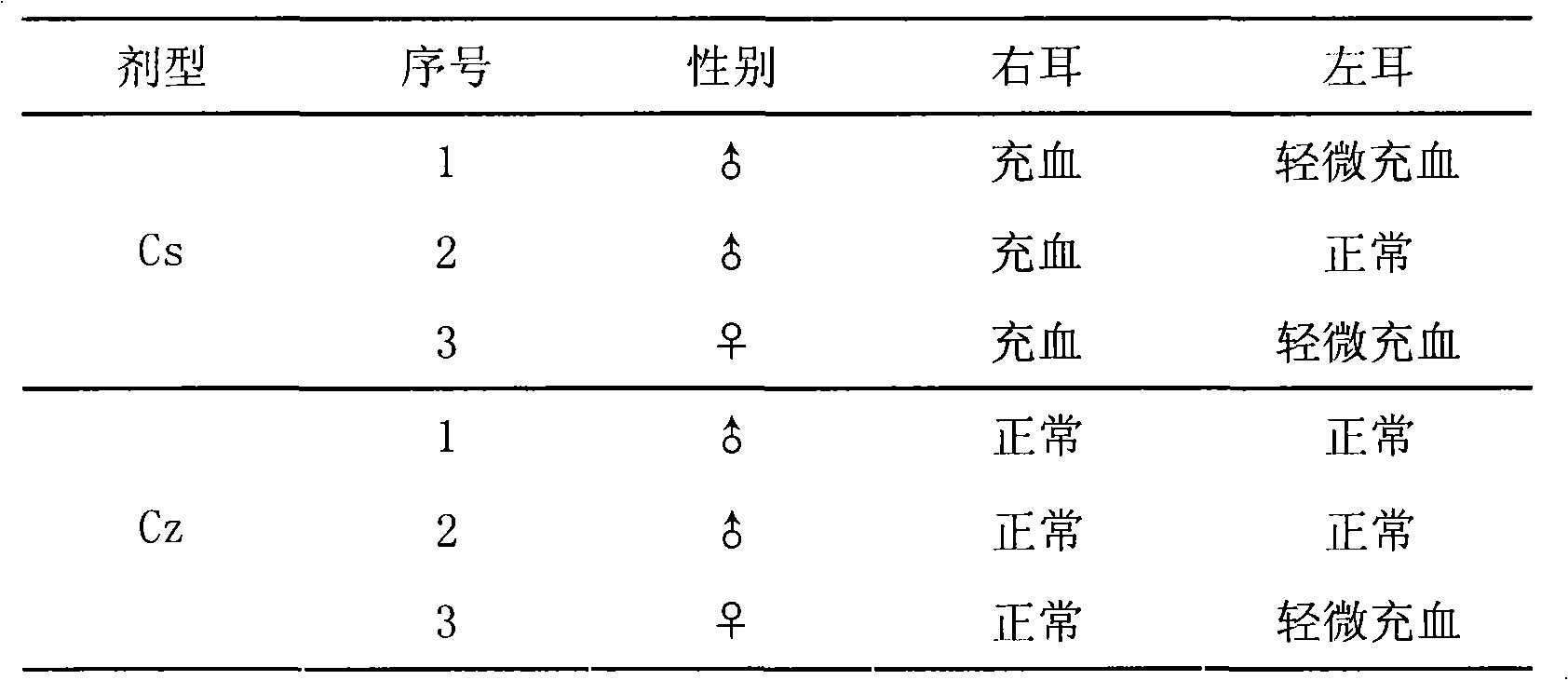
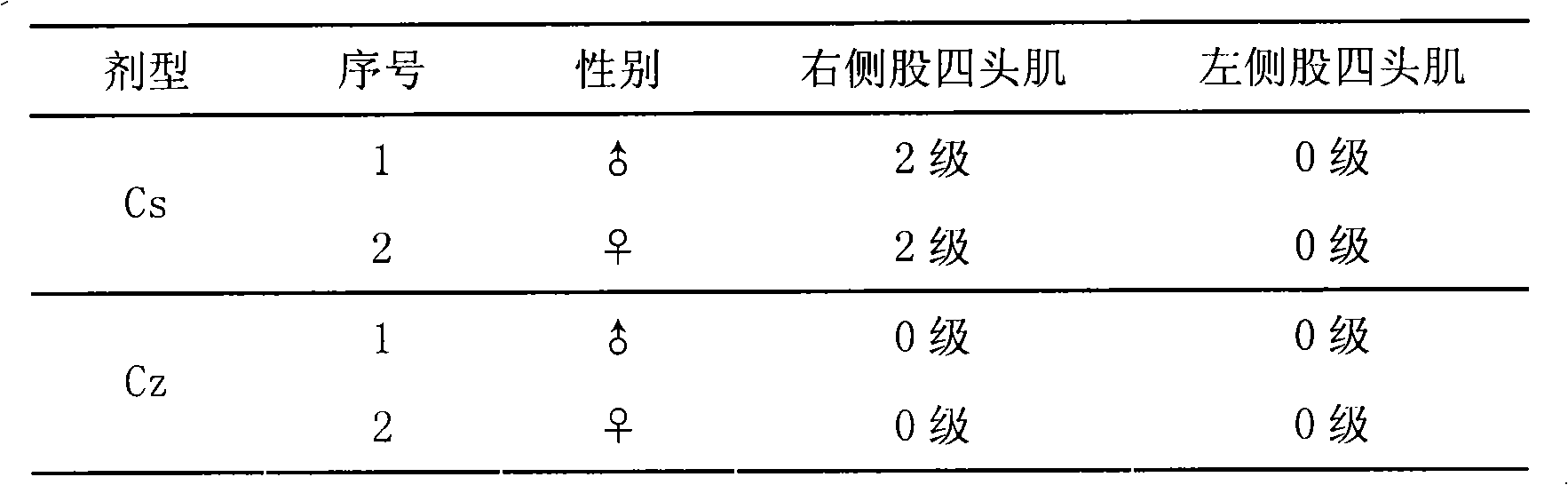
Medicine composition for injecting triamcinolone acetonide palmitate lipid microsphere and preparation method thereof
A technology of palmitate and lipid microspheres, applied in the field of medicine, can solve the problems of drug precipitation, pain, human toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] The composition and preparation process of each component in the pharmaceutical composition for injection of triamcinolone palmitate lipid microspheres per 100ml
[0131] composition:
[0132] Triamcinolone Palmitate 10%
[0133] Soybean Oil 25%
[0134] Medium Chain Fatty Acid Triglycerides 15%
[0135] Soy Lecithin 6.4%
[0136] Tween-80 0.8%
[0137] F68 1.0%
[0138] Glycerin 6.5%
[0139] EDTA0.5%
[0140] Sodium Oleate 0.1%
[0141] Appropriate amount of pH adjuster
[0142] The rest is water for injection.
[0143] Preparation:
[0144] (1) Mix the prescribed amount of glycerin, Tween-80, F68, EDTA, sodium oleate with water for injection preheated to 80°C, and stir until the ingredients are dissolved to obtain the aqueous phase;
[0145] (2) adding the prescribed amount of soybean lecithin and triamcinolone acetonide palmitate to the oily medium of soybean oil and medium-chain fatty acid triglycerides and mixing, heating to 80° C., and magnetically sti...
Embodiment 2
[0151] The composition and preparation process of each component in the pharmaceutical composition for injection of triamcinolone palmitate lipid microspheres per 100ml
[0152] Triamcinolone Acetonide Palmitate 0.01%
[0153] Soybean Oil 2.0%
[0154] Medium Chain Fatty Acid Triglycerides 1.5%
[0155] Soy Lecithin 0.5%
[0156] Tween-80 0.05%
[0157] F68 0.05%
[0158] Glycerin 2.25%
[0159] EDTA0.02%
[0160] Sodium Oleate 0.01%
[0161] Appropriate amount of pH adjuster
[0162] The rest is water for injection.
[0163] Preparation:
[0164] Use the components and their dosages of this example to prepare according to the same preparation method as in Example 1.
Embodiment 3
[0166] The composition and preparation process of each component in the pharmaceutical composition for injection of triamcinolone palmitate lipid microspheres per 100ml
[0167] Triamcinolone Acetonide Palmitate 0.2%
[0168] Soybean Oil 7.5%
[0169] Medium Chain Fatty Acid Triglycerides 5.0%
[0170] Soy Lecithin 3.5%
[0171] Tween-80 0.8%
[0172] F68 0.6%
[0173] Glycerin 0.5%
[0174] Sodium Oleate 0.06%
[0175] Appropriate amount of pH adjuster
[0176] The rest is water for injection.
[0177] Preparation:
[0178] The components and their dosages of this example were used to prepare according to the same preparation method as that of Formulation Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com