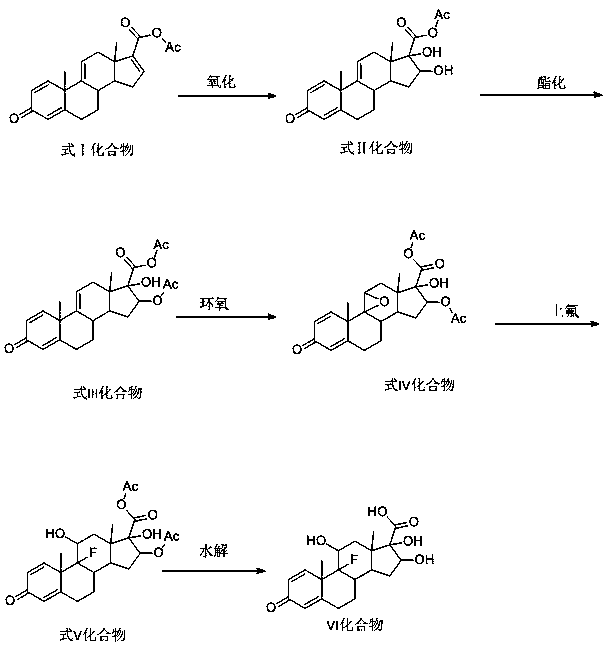
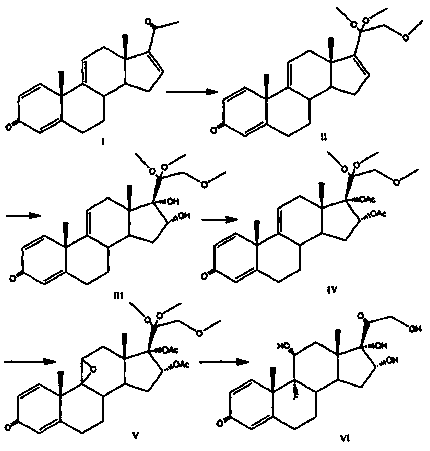
Triamcinolone preparation method
A compound and organic solvent technology, applied in the field of preparation of triamcinolone, can solve the problems of cumbersome product purification, and achieve the effects of excellent yield and cost, high yield and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the triamcinolone involved in this embodiment specifically comprises the following steps:
[0032] (1) Oxidation reaction: Add 30g of the compound of formula I and 30ml of formic acid into 600ml of methanol at a temperature of -10°C, and carry out the oxidation reaction under the action of excess potassium permanganate. After the TLC test is qualified, add excess sodium bisulfite Remove the acid and oxidizing agent from the aqueous solution, then concentrate, add water to precipitate the product, control the temperature during the precipitation process below 5°C, finally centrifuge the material, and dry to obtain 30.3g of the compound of formula II, with a yield of 101%;
[0033] (2) Esterification reaction: Add 30.3g of the compound of formula II obtained in step (1) into 303ml of chloroform, carry out esterification reaction with 30.3ml of acetic anhydride, heat and reflux with steam for 14-16 hours, and TLC detects that there is no raw materi...
Embodiment 2
[0040] The preparation method of the triamcinolone involved in this embodiment specifically comprises the following steps:
[0041] (1) Oxidation reaction: Add 30g of formula I compound and 60ml perchloric acid into 1200ml of dichloromethane at -5°C, carry out oxidation reaction under the action of excess potassium dichromate, after TLC detection is qualified, add excess sodium sulfite Remove the acid and oxidizing agent from the aqueous solution, then concentrate, add water to precipitate the product, control the temperature during the precipitation process below 5°C, finally centrifuge the material, and dry to obtain 30 g of the compound of formula II, with a yield of 100%;
[0042] (2) Esterification reaction: add 30g of the compound of formula II obtained in step (1) into 300ml of dichloromethane, carry out esterification reaction with 30ml of acetic acid, heat and reflux with steam for 14-16 hours, the end of the reaction when there is no raw material point detected by TLC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com