Triamcinolone biodegradable maltose microneedle array and preparation method thereof
A technology of microneedle array and triamcinolone acetonide, which can be used in drug delivery, drug combination, and pharmaceutical formulation, and can solve problems such as unbearable pain, imbalance, and multi-point injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 Preparation of triamcinolone acetonide biodegradable maltose microneedle array by stepwise controlled pulling method
[0083] (1) Dissolve 10 mg of commercially available maltose powder and 10 μL of deionized water at 110°C for 10 min, then spread the liquid maltose on a circular stainless steel substrate, and quickly add 1 mg of triamcinolone acetonide (Tianjin Pacific Chemical Pharmaceutical Co., Ltd.) powder , mix evenly, cool down to 103°C and spread the mixture to a thickness of 122±13.5μm.
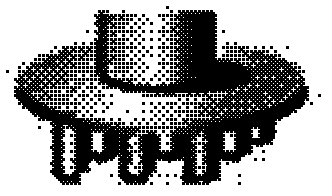
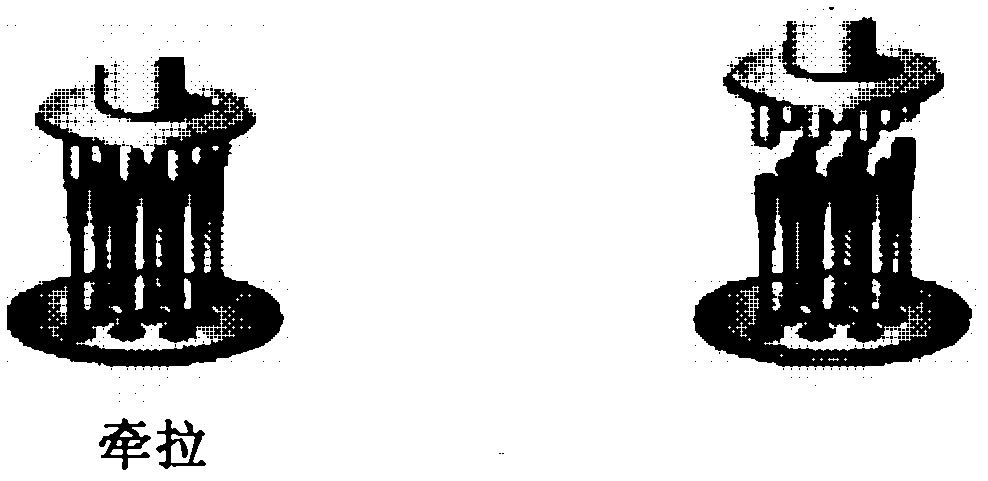
[0084] (2) Put the prepared stainless steel column frame (such as figure 1 shown) adhered to the spread maltose, and microneedles with different needle shapes could be prepared according to pulling the maltose at different temperatures.
[0085] (3) a. Baseball rod-shaped microneedles: Control the temperature at 100°C, when the maltose matrix is in a glass state, pull the maltose at a speed of 400μm / s for 3s, cool down to 60°C, cool and solidify the microneedles, ...
Embodiment 2
[0088] Example 2 Preparation of triamcinolone acetonide biodegradable maltose microneedle array by micromolding method
[0089] (1) Dissolve 10 mg maltose and 10 μL deionized at 110° C. for 10 min, then add 1 mg powdered triamcinolone acetonide and mix well.
[0090] (2) Pour the liquid mixture into a conical mold, cover with a 300g stainless steel plate, and cool slowly to make it solidify. The steel plate is then removed, separating the solid microneedles from the mold.
Embodiment 3
[0091] Example 3 Pain Sensation Experiment of Triamcinolone Maltose Microneedle Array
[0092] (1) Case source: 12 scar patients, aged 22-53 years, with an average age of (37.59±8.04) years, including 6 males and 6 females.
[0093] (2) Administration method: local injection of triamcinolone acetonide injection and triamcinolone acetonide maltose microneedle array administration respectively.
[0094] (3) The patient's pain score standard is shown in Table 1:
[0095] Table 1
[0096] It's a sharp pain
more painful
pain
a little pain
painless
10-8
8-6
6-4
4-2
2-0
[0097] (4) Statistical method
[0098] The data are represented by mean ± standard deviation (x ± SD), and the statistical analysis is carried out with SAS6.12 statistical analysis software, and the data of different administration methods are analyzed by paired t test, and P<0.01 means that the difference is statistically significant.
[0099] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com