Limit size lipid nanoparticles and related methods
a technology of lipid nanoparticles and limit size nanoparticles, which is applied in the direction of granular delivery, flow mixers, powder delivery, etc., can solve the problems of limited sample contamination, sample degradation, and inability to produce systems smaller than approximately 50 nm,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
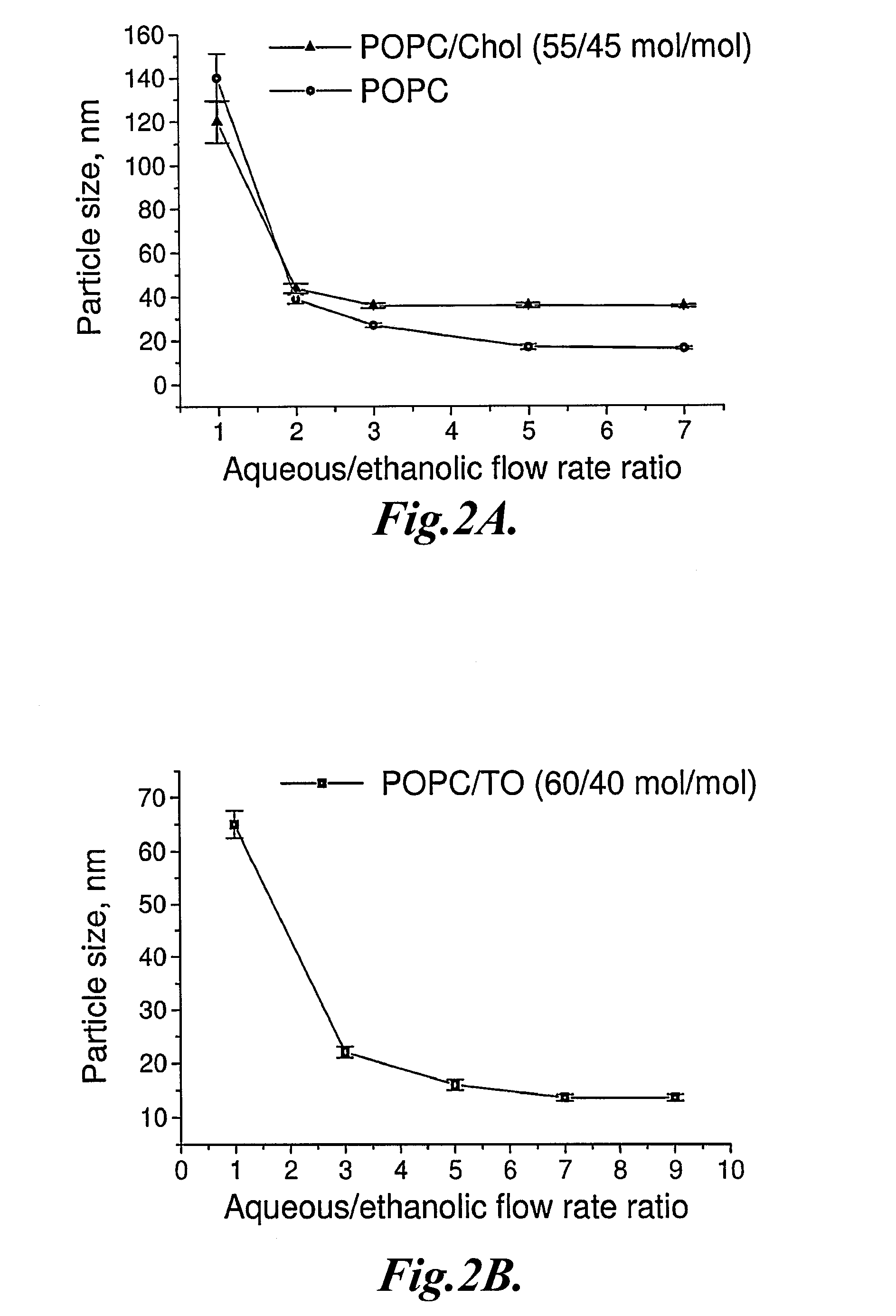
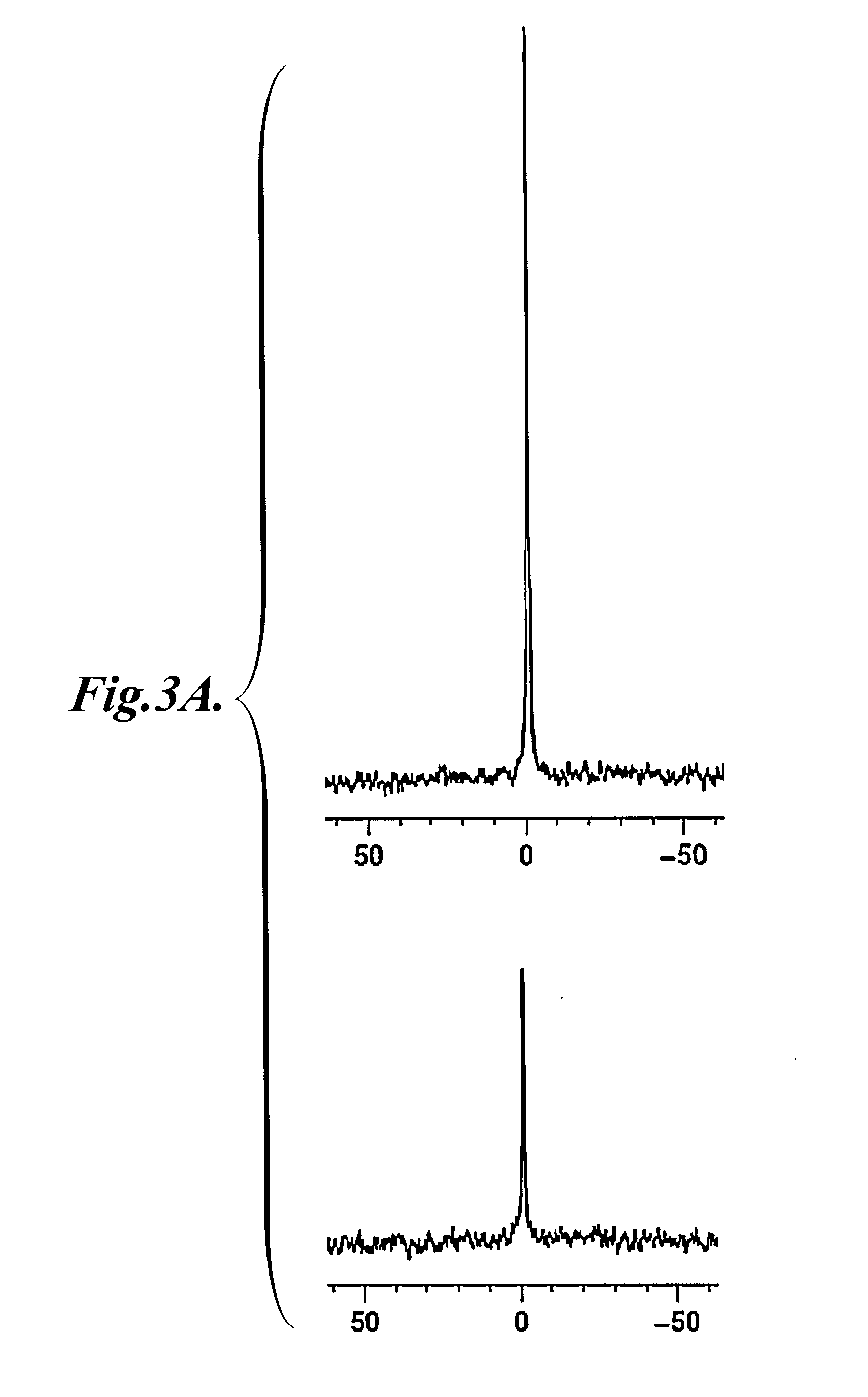
Preparation and Characterization of Representative LNP
[0187]In this example, the preparation and characterization of representative LNP are described.
[0188]Lipids and Chemicals.
[0189]1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was obtained from Avanti Polar Lipids (Alabaster, Ala.). 1,2,3-Tri(cis-9-octadecenoyl) glycerol (glyceryl trioleate, TO), cholesterol (Chol), sodium chloride, ammonium sulfate, and doxorubicin hydrochloride were from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada).
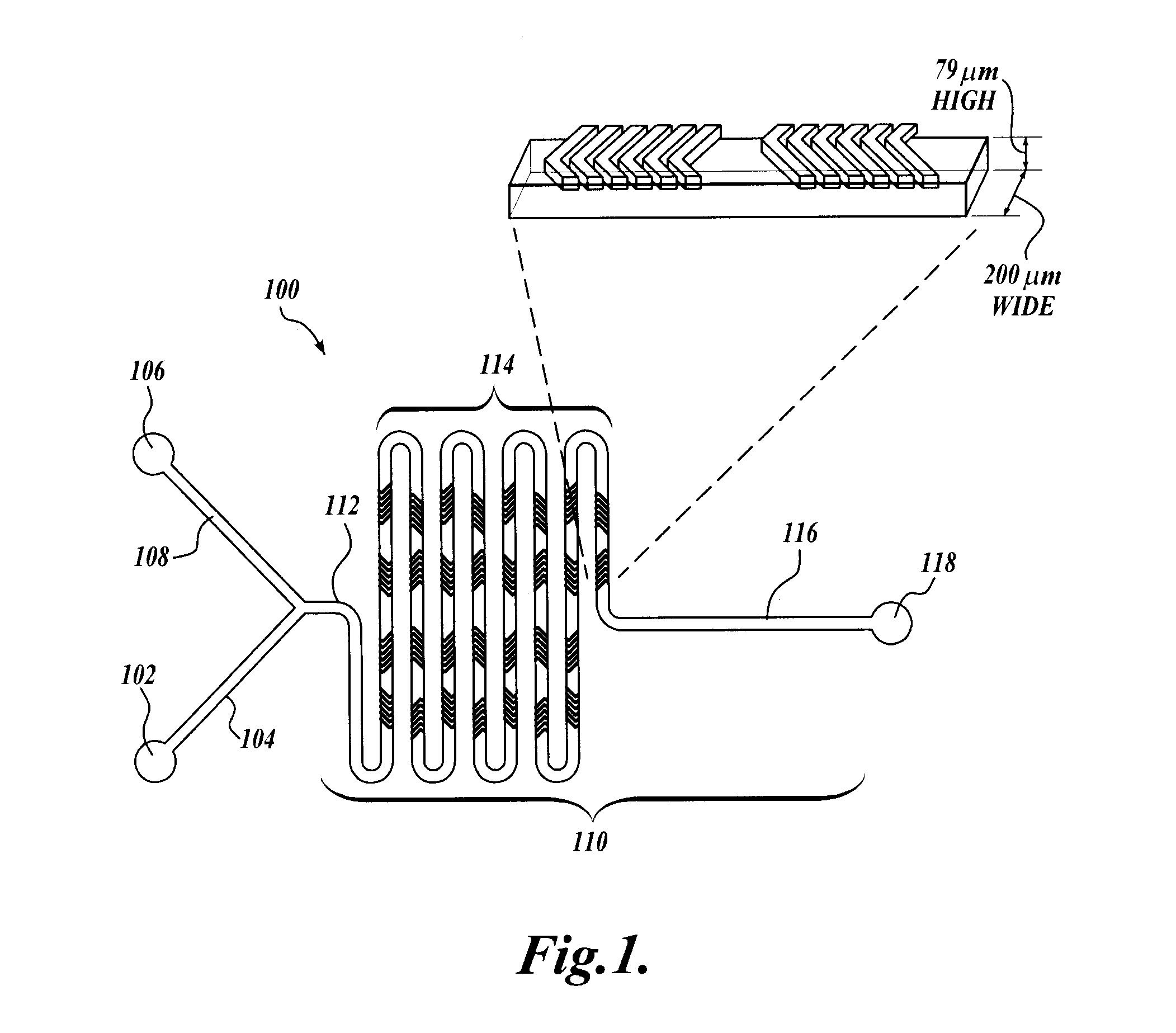
[0190]Micromixer Design and Fabrication.
[0191]The micromixer was a chaotic mixer for continuous flow systems with the layout based on patterns of asymmetric grooves on the floor of the channel (staggered herringbone design) that induce a repeated sequence of rotational and extensional local flows thus inducing rapid mixing of the injected streams. The device was produced by soft lithography, the replica molding of microfabricated masters in elastomer. The device features a 200 μm wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com