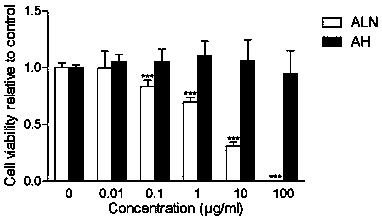
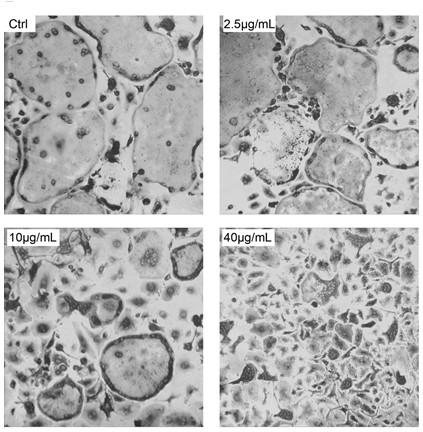
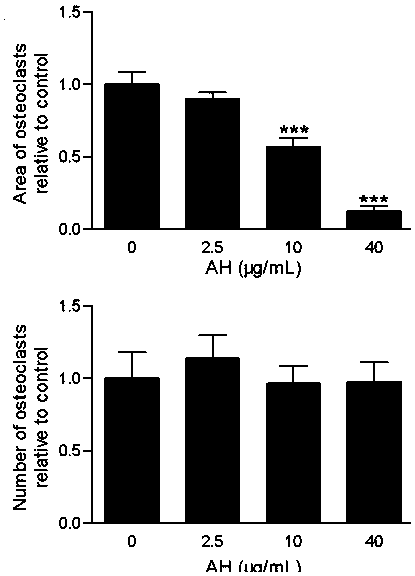
Heparin alendronate sodium conjugate synthetic method and drugs
A technology for the synthesis of sodium alendronate and sodium allenphosphate, which is applied in the field of synthesis of heparin-alendronate sodium conjugates, can solve the problems of inability to produce on a large scale, high preparation cost, cumbersome operation, etc., and achieve convenience for large-scale production and high synthesis efficiency High, simple synthesis process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this example, heparin alendronate sodium conjugates were first synthesized through the following process:
[0037] 1) Dissolve 23g of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) in 300ml of tetrahydrofuran (THF);
[0038] 2) Add 13.2ml of 4-methylmorpholine (NMM) into the solution in step 1), and stir vigorously for 1 hour; collect the solid by filtration and wash the solid with THF 5 times, and dry it in vacuum for 48 hours to obtain the DMT-MM condensing agent;
[0039] 3) Dissolve 1g of heparin and 0.874g of the DMT-MM condensing agent prepared in step 2) in 50ml of ultrapure water, and stir for 1h;
[0040] 4) Dissolve 2g of alendronate sodium in 100ml of ultrapure water;
[0041] 5) Add alendronate sodium solution into the mixed solution of heparin and condensing agent in step 3), react for 24 hours, dialyze the solution through a dialysis bag with a molecular weight of 300 for 48 hours, and change the water every 4 hours, filter out the condensing agent and ...
Embodiment 2
[0043]Synthesis of heparin alendronate sodium conjugates in this example:
[0044] 1) Dissolve 40g of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) in 400ml of tetrahydrofuran (THF);
[0045] 2) Add 22ml of 4-methylmorpholine (NMM) to the solution in step 1), and stir vigorously for 1.5h; collect the solid by filtration and wash the solid with THF 5 times, and dry it in vacuum for 48h to obtain the DMT-MM condensate of this example. mixture;
[0046] 3) Dissolve 1.5g of heparin and 1.4g of the DMT-MM condensing agent obtained in step 2) in 50ml of ultrapure water, and stir for 1.5h;
[0047] 4) Dissolve 3g of alendronate sodium in 200ml of ultrapure water;
[0048] 5) Add the alendronate sodium solution in step 4) to the mixed solution of heparin and DMT-MM condensing agent in step 3), react for 24-48 hours, dialyze the solution through a dialysis bag with a molecular weight of 800 for 60 hours, and The water was changed every 4 hours, the condensing agent and excess reactan...
Embodiment 3
[0050] In this example, heparin alendronate sodium conjugates were first synthesized
[0051] 1) Dissolve 55g of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) in 500ml of tetrahydrofuran (THF);
[0052] 2) Add 30ml of 4-methylmorpholine (NMM) into the solution in step 1), and stir vigorously for 2 hours; collect the solid by filtration, wash the solid with THF for 5 times, and dry it in vacuum for 48 hours to obtain the DMT-MM condensing agent;
[0053] 3) Dissolve 2g of heparin and 2g of the DMT-MM condensing agent in step 2) in 50ml of ultrapure water, and stir for 2 hours;
[0054] 4) Dissolve 5g of alendronate sodium in 300ml of ultrapure water;
[0055] 5) Add the alendronate sodium solution in step 4) to the mixed solution of heparin and condensing agent in step 3), react for 48 hours, dialyze the solution for 72 hours through a dialysis bag with a molecular weight of 1000, and change the water every 4 hours , filter out the condensing agent and excess reactants, and fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com