Multi-unit bowel dissolvable preparation of compound alendronate sodium and vitamin D3 and preparation method thereof
A technology for compound alendronate sodium and alendronate sodium, which can be applied to medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., and can solve problems such as increased dose, low bioavailability, pain, etc. , to avoid irritation, increase contact area, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
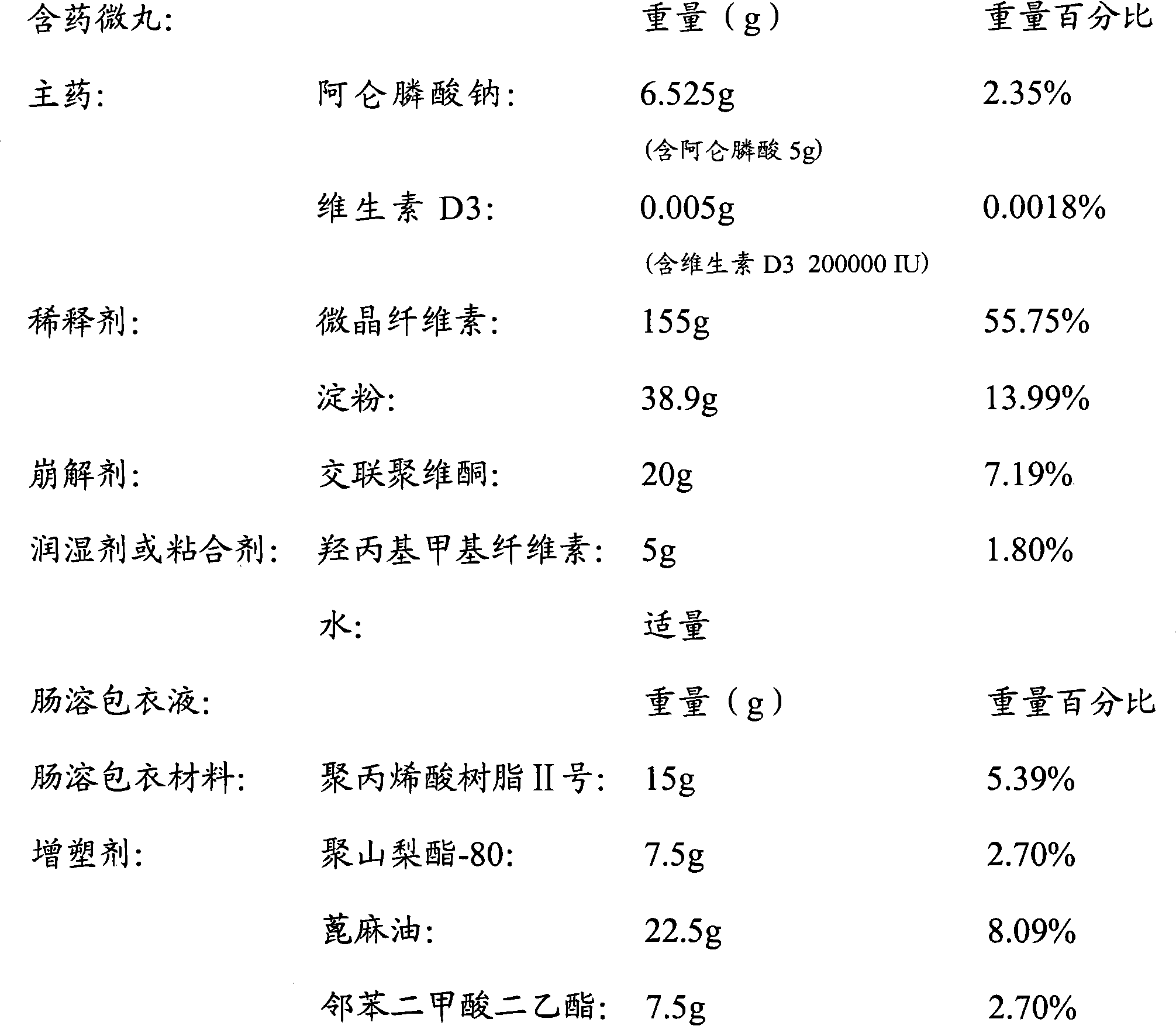
[0032] Prepare materials according to the following formula: (1000 capsules)
[0033]
[0034] Weigh alendronate sodium, vitamin D3, diluent, and disintegrant through a 100-mesh sieve according to the above formula, mix well, add an appropriate amount of hydroxypropyl methylcellulose solution, prepare pellets and dry them;
[0035] Dissolve the enteric-coated material in an appropriate amount of ethanol, and add a plasticizer to adjust the film-forming properties of the enteric-coated solution;
[0036] The prepared drug-containing pellets are placed in a fluidized bed or other coating equipment, and the coating liquid is sprayed while blowing hot air to obtain enteric-coated pellets.
Embodiment 2
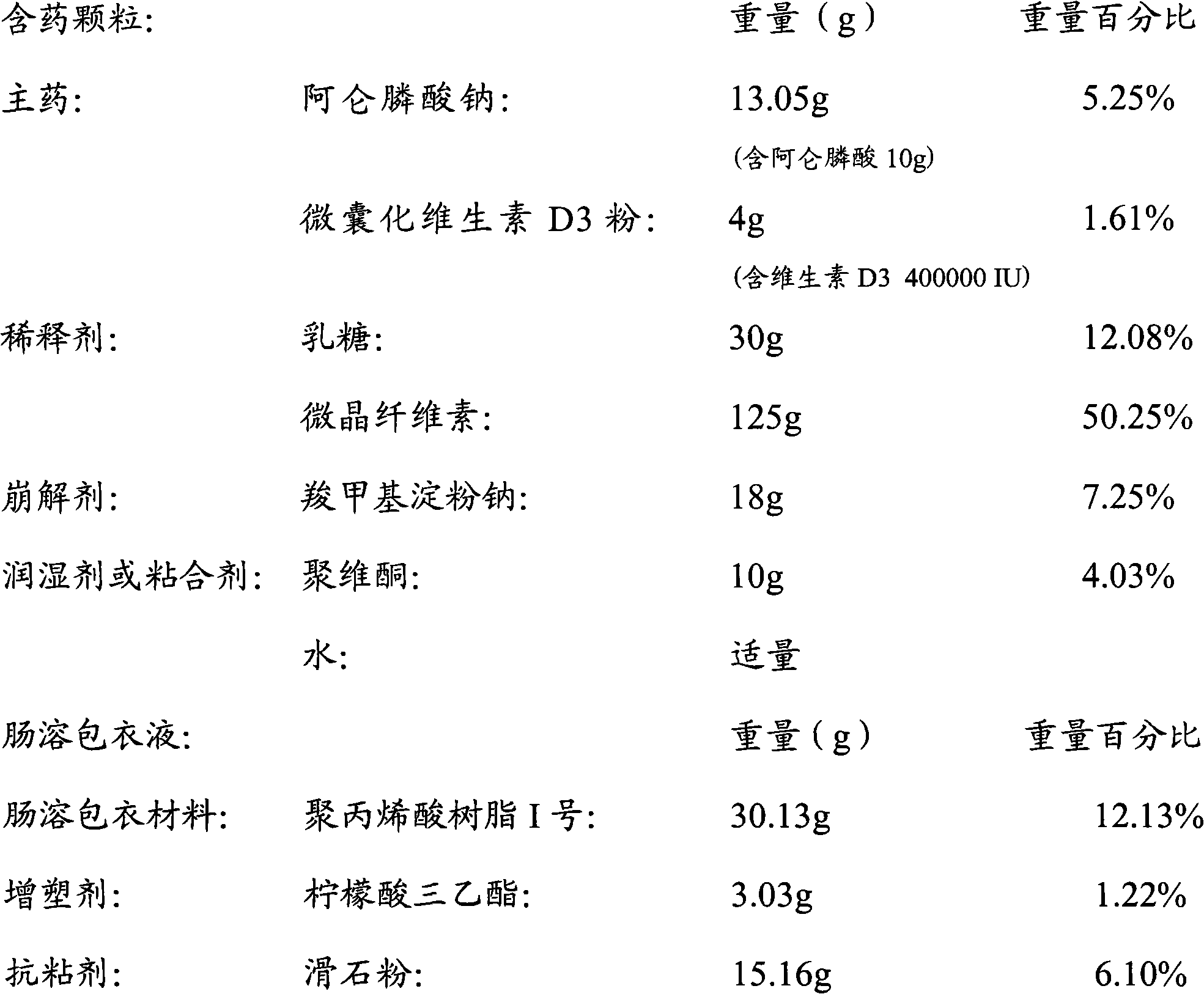
[0038] Prepare materials according to the following formula: (1000 capsules)
[0039]
[0040] According to the above formula, take alendronate sodium, microencapsulated vitamin D3 powder, diluent, and disintegrant through a 100-mesh sieve, mix well, add an appropriate amount of povidone solution, prepare granules and dry them;
[0041] Disperse the enteric material in an appropriate amount of water, add a plasticizer to adjust the film-forming properties of the enteric coating solution, and add an anti-adhesive agent to adjust the viscosity of the enteric coating solution;
[0042] The prepared drug-containing granules are placed in a fluidized bed or other coating equipment, and the coating liquid is sprayed while blowing hot air to obtain enteric-coated granules.
Embodiment 3
[0044] Prepare materials according to the following formula: (1000 capsules)
[0045]
[0046] Weigh alendronate sodium, vitamin D3, diluent, and disintegrant through a 100-mesh sieve according to the above formula, mix well, add an appropriate amount of water, prepare pellets and dry them;
[0047] Dissolving the enteric material in an appropriate amount of ammonia water with a pH of 9, adding a plasticizer to adjust the film-forming properties of the enteric coating solution, and adding an anti-adhesive agent to adjust the viscosity of the enteric coating solution;
[0048] The prepared drug-containing pellets are placed in a fluidized bed or other coating equipment, and the coating liquid is sprayed while blowing hot air to obtain enteric-coated pellets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com