Composite type medical biological bone cement, preparation method and application thereof
A composite bone cement technology, applied in medical science, prosthesis, etc., can solve problems such as high elastic modulus of bone cement, nerve tissue damage, non-degradable absorption, etc., to inhibit bone resorption, promote new bone formation, and prepare Method quick and easy results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
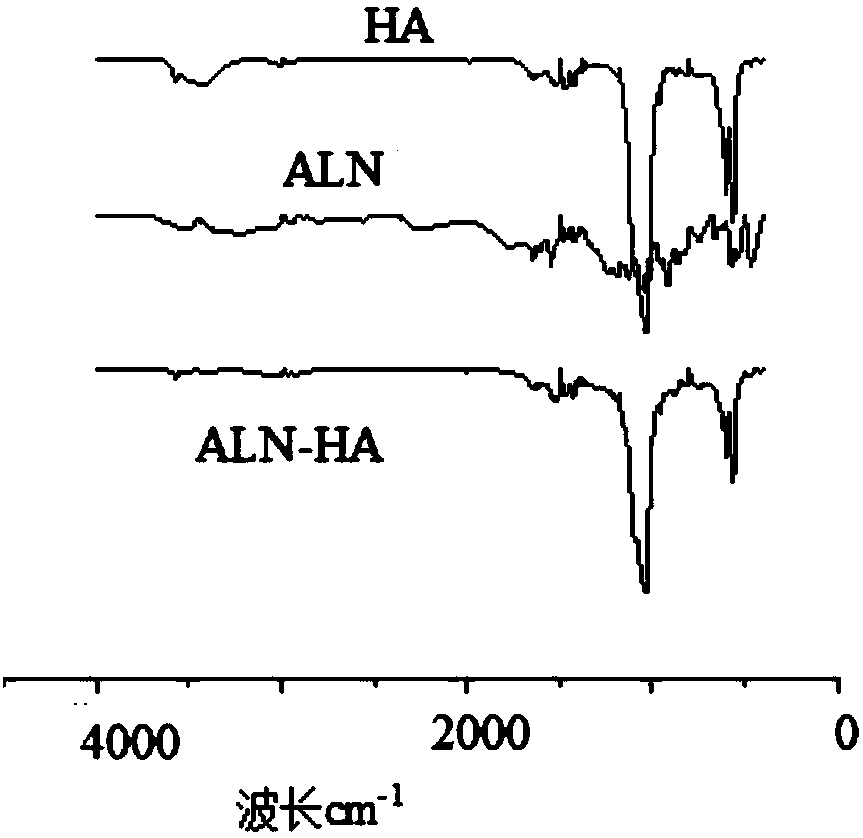
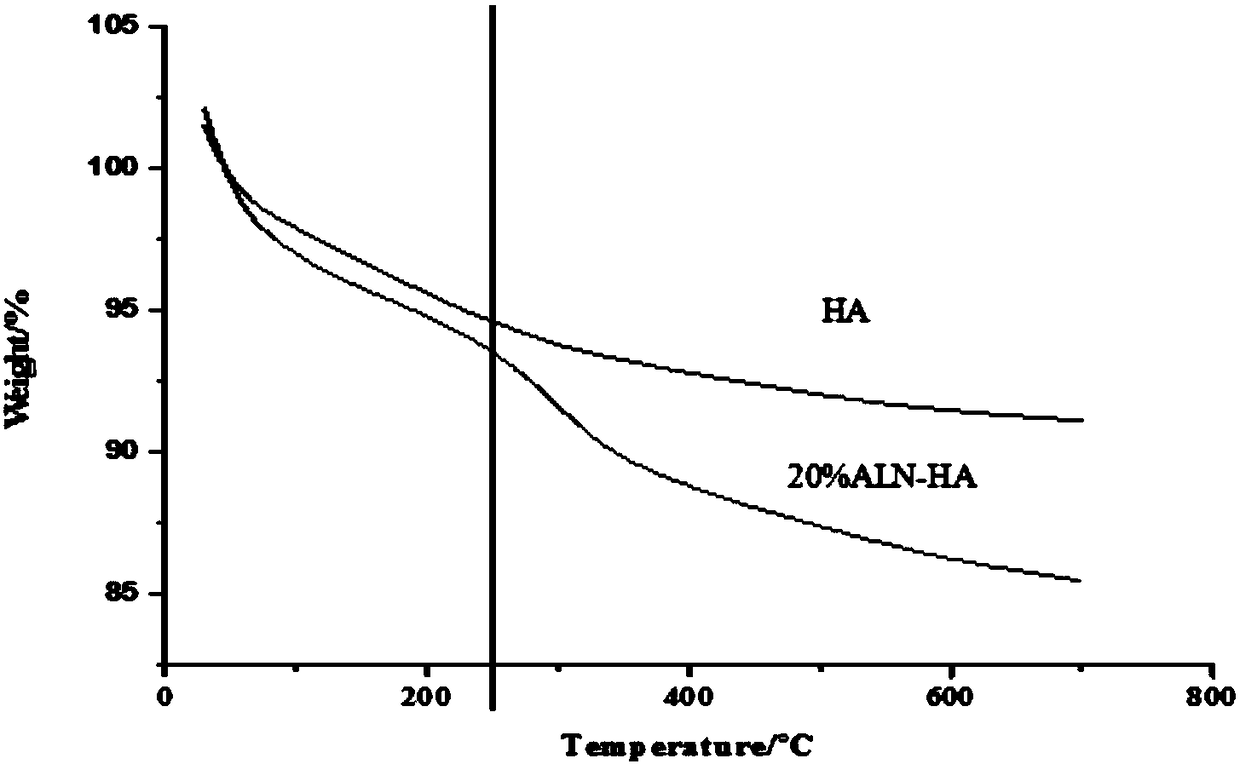
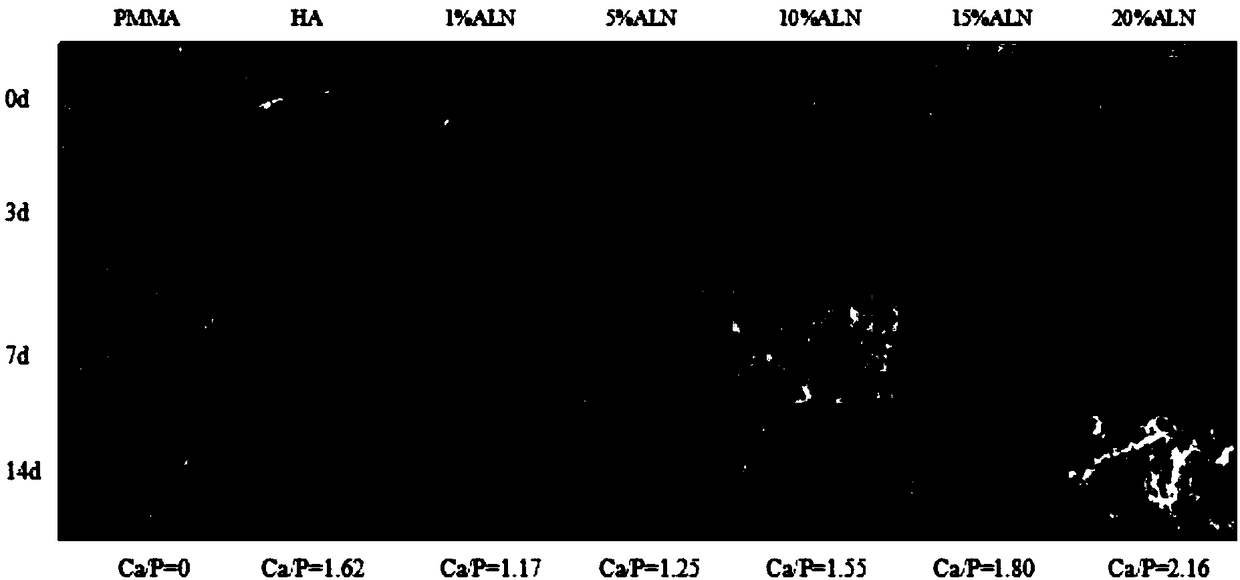
[0023] A composite medical bio-bone cement, comprising a powder part and a liquid part with a weight ratio of 1:1, and the powder part, in parts by weight, comprises sodium alendronate-nano hydroxyapatite (ALN-HA ) powder 0.4g, polymethyl methacrylate powder (PMMA) 0.6g, dibenzoyl peroxide (BPO) 0.03g that the number average molecular weight is 500,000-700,000; Limestone powder (ALN-HA) was prepared as follows: Alendronate sodium (ALN) dissolved in ultrapure water was added to nano-hydroxyapatite (nHA), and the mixture of alendronate sodium and nano-hydroxyapatite The mass ratio is 0.01-0.2, stirred at 90°C for 5 hours, freeze-dried for 72 hours, and ground to obtain the target product. For its infrared spectrum, see figure 1 ; When the mass ratio of sodium alendronate and nano-hydroxyapatite is 0.2, that is, when the degree of modification of ALN in ALN-HA is 20%, the experimental thermogravimetric analysis results are as follows: figure 2 Shown; Described liquid portion co...
Embodiment 2
[0028] Referring to Example 1, the difference is that the weight ratio of the powder part to the liquid part is 1:1.5, 0.45 g of alendronate sodium-nanometer hydroxyapatite (ALN-HA) powder, and a number average molecular weight of 500,000-700,000 Polymethyl methacrylate powder (PMMA) 0.45g, dibenzoyl peroxide (BPO) 0.04g.
[0029] Compressive strength: According to the ISO5833 bone cement standard, a 12mm×6mm cylindrical specimen was prepared, and after curing at a constant temperature of 37°C for 24 hours, the compressive strength was measured with an AGS-10KN universal testing machine (AGS-10KN, Universal Testing Machines, Shimadzu, Japan) . The results show that the mechanical properties of the bone cement obtained by the present invention are up to the standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com