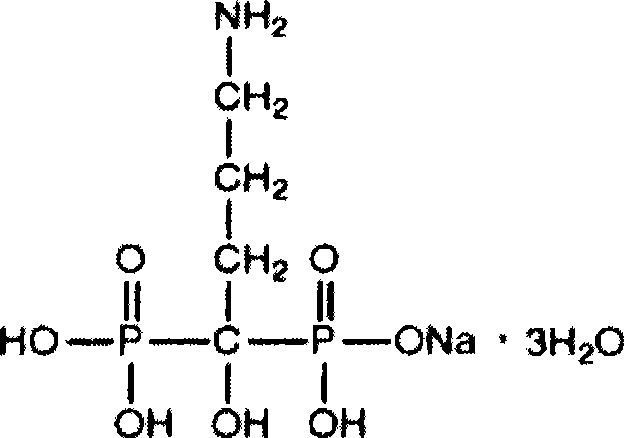
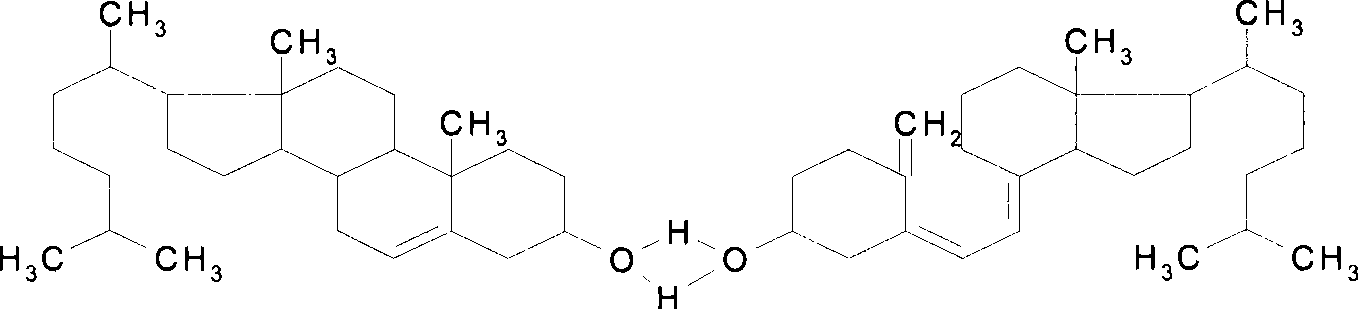
Pharmaceutical preparation containing alendronate sodium and cholecalciferol-cholesterol
A technology of alendronate sodium and cholvitin, which is applied in the field of pharmaceutical preparations containing alendronate sodium and cholvitin, can solve the problems of oral dosage form limitation, inconvenient production and storage, unstable raw material of vitamin D3, etc. The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
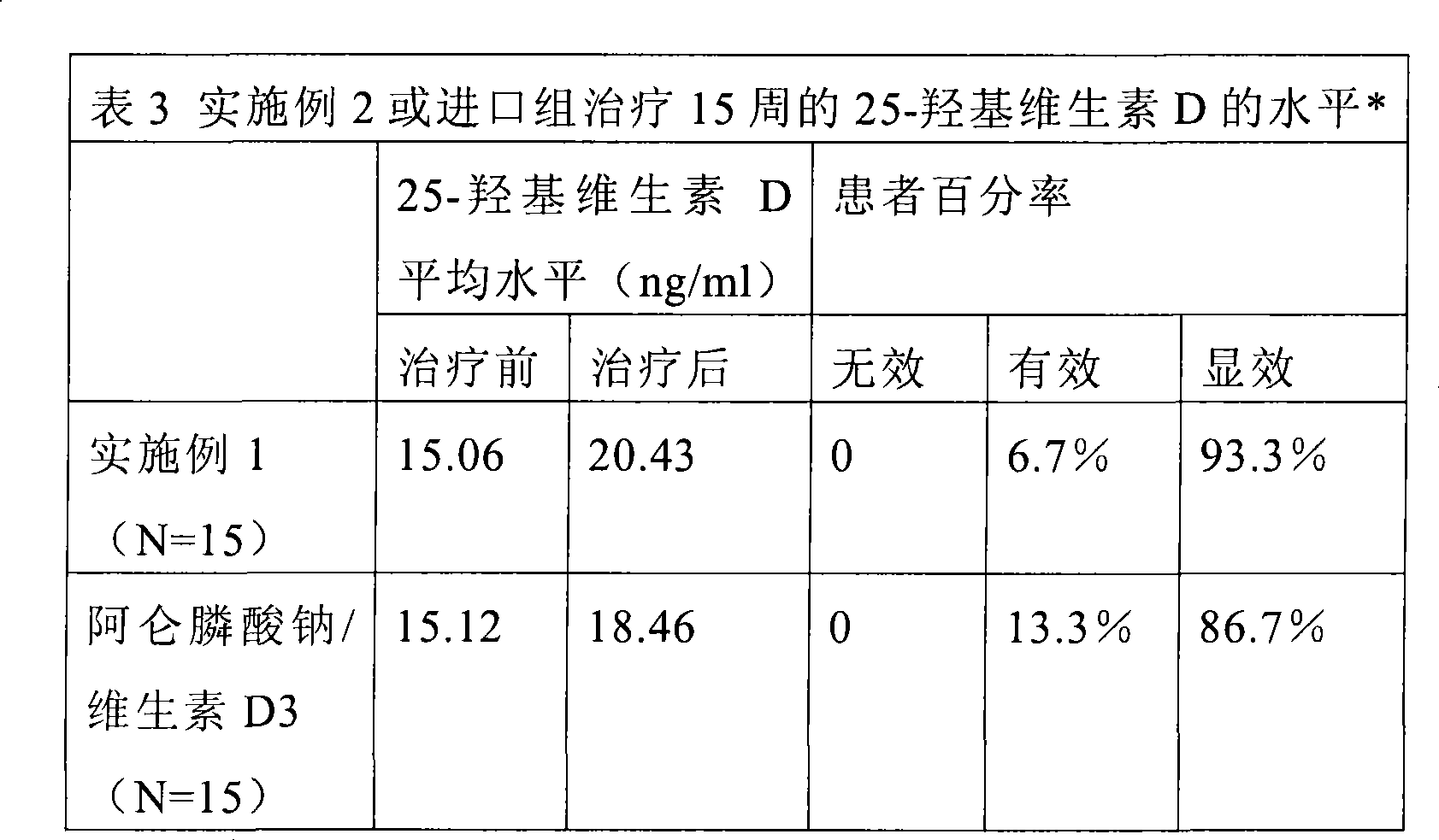
[0072] Example 1: Alendronate Sodium Cholevetin Ordinary Tablets or Capsules
[0073] Specifications: Alendronate Sodium 70mg / 0.14mg Cholevetin (Equivalent to 2800IU Vitamin D3)
[0074] And alendronate sodium 70mg / 0.28mg cholevetin (equivalent to 5600IU vitamin D3)
[0075] prescription:
[0076] Name of raw material Dosage per tablet source monosodium alendronate trihydrate
Salt
Salt 91.37mg (equivalent to each tablet containing
70mg of alendronate)
Shaanxi Hanjiang Pharmaceutical Co., Ltd.
Cilivitin 0.14mg or 0.28mg Chongqing Kerui Pharmaceutical Co., Ltd. microcrystalline cellulose
(pH301) 30mg
Asahi Kasei Corporation
anhydrous lactose 43mg new zealand lactose co. Croscarmellose Sodium 15mg Anhui Shanhe Pharmaceutical Excipients Co., Ltd. sucrose 10mg Huzhou Prospect Pharmaceutical Co., Ltd. colloidal silica 0.5mg Huzhou Prospect Pharmaceutical Co., Ltd. Magnesi...
Embodiment 2
[0085] Example 2: Alendronate Sodium Cholevetin Oral Emulsion
[0086] Specifications: 10ml: alendronate sodium 70mg / 0.14mg cholevetin (equivalent to 2800IU vitamin D3)
[0087] And 10ml: alendronate sodium 70mg / 0.28mg cholevetin (equivalent to 5600IU vitamin D3)
[0088] prescription:
[0089] Name of raw material Amount per bottle source monosodium alendronate trihydrate
Salt
Salt 91.37mg (equivalent to each tablet containing
70mg of alendronate)
Shaanxi Hanjiang Pharmaceutical Co., Ltd.
Cilivitin 0.14mg or 0.28mg Chongqing Kerui Pharmaceutical Co., Ltd. tea oil
0.75g
Juxing Grain and Oil Medicalization in Longyou County, Zhejiang Province
Industrial Co., Ltd. Tween 80
0.6g
Zhejiang Longyou Juxing Grain and Oil Pharmaceutical Chemicals
limited company Span-85 0.15g Shanghai Shenyu Pharmaceutical Chemical Co., Ltd. B.H.A (tert-butyl-4-
Hydroxyanisole) 0.01g
Shanghai S...
Embodiment 3
[0096] Embodiment 3: Alendronate Sodium Cholevetin Dropping Pills
[0097] Specifications: Alendronate Sodium 70mg / 0.14mg Cholevetin (Equivalent to 2800IU Vitamin D3)
[0098] And alendronate sodium 70mg / 0.28mg cholevetin (equivalent to 5600IU vitamin D3)
[0099] prescription:
[0100] Name of raw material Amount per bottle source Alendronic acid trihydrate
sodium salt 91.37mg (equivalent to each tablet containing
70mg of alendronate) Shaanxi Hanjiang Pharmaceutical Co., Ltd.
Cilivitin 0.14mg or 0.28mg Chongqing Kerui Pharmaceutical Co., Ltd. L-cysteine hydrochloride 0.25mg Shanghai Ajinomoto Co., Ltd. PEG6000 900mg Shanghai Pudong Gaonan Chemical Factory
[0101] Preparation method:
[0102] (1) Take 900g of PEG 6000 oil bath and heat it to 135°C, then add 0.14g of cholevetin, 91.37g of alendronate monosodium trihydrate and 0.3g of L-cysteine hydrochloride into PEG6000 while stirring Make all dissolved...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com