Compound alendronate sodium vitamine D3 orally disintegrating tablets and preparation method thereof
A technology of oral disintegrating tablet and alendronate sodium, which is applied in the field of compound alendronate sodium vitamin D3 oral disintegrating tablet and its preparation, and can solve the problem that no related documents and patents of prulifloxacin oral disintegrating tablet have been seen. reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
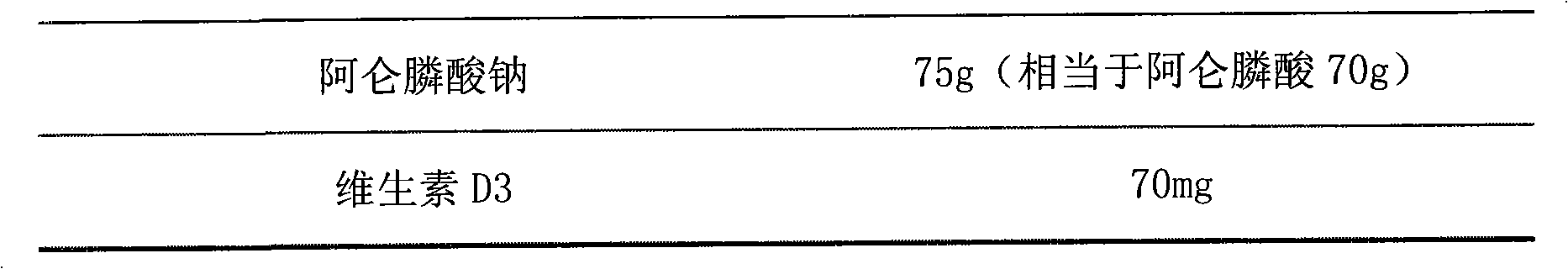
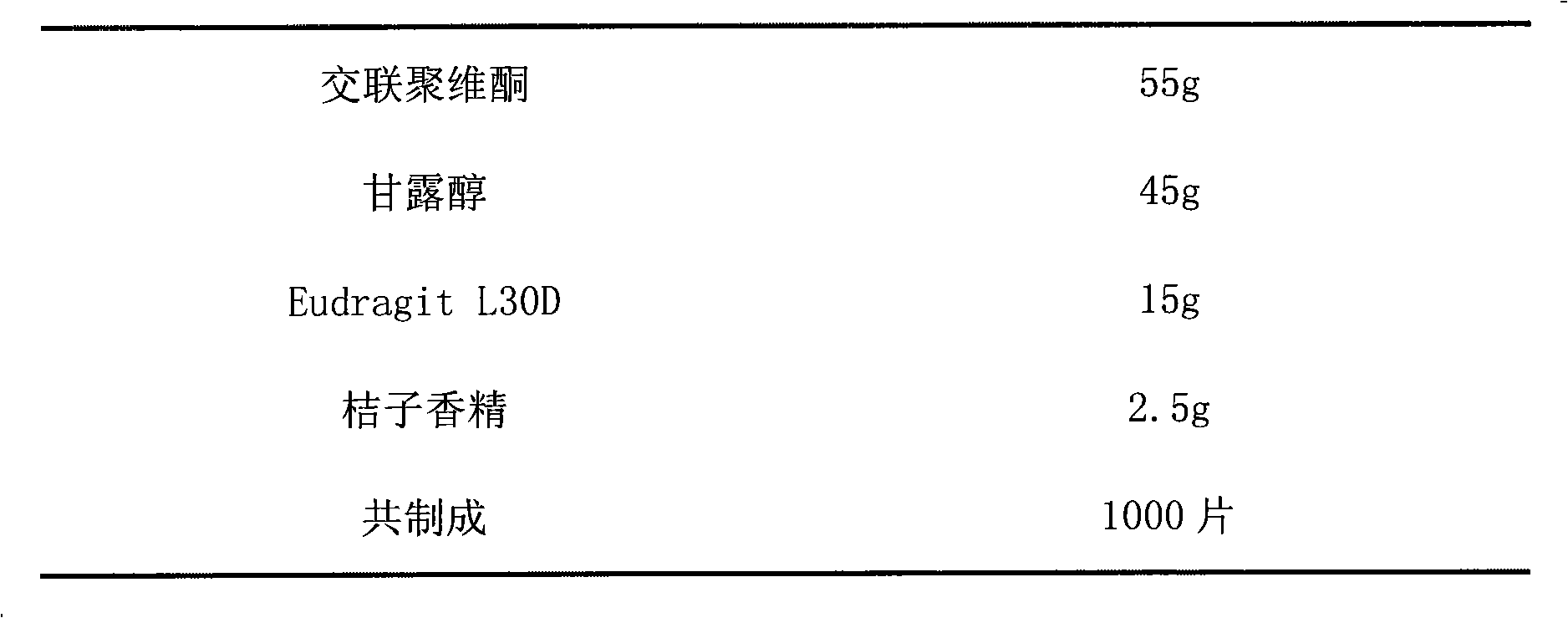
[0021] prescription:
[0022]
[0023]
[0024] Preparation:
[0025] Add alendronate sodium vitamin D3 into Eudragit L30D suspension, add appropriate amount of water, stir evenly, add crospovidone, mannitol, orange essence, stir while adding, after forming a suspension, pour in In a suitable mold, freeze-dried, pressed and sealed, and packaged.
Embodiment 2
[0027] prescription:
[0028]
[0029] Preparation:
[0030] Mix alendronate sodium vitamin D3, pregelatinized starch, mannitol, and lactose that have passed through an 80-mesh sieve evenly according to the method of equal addition, and mix protein sugar and pineapple essence with the above mixture, add micronized silica gel and mix evenly , the powder can be directly compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com