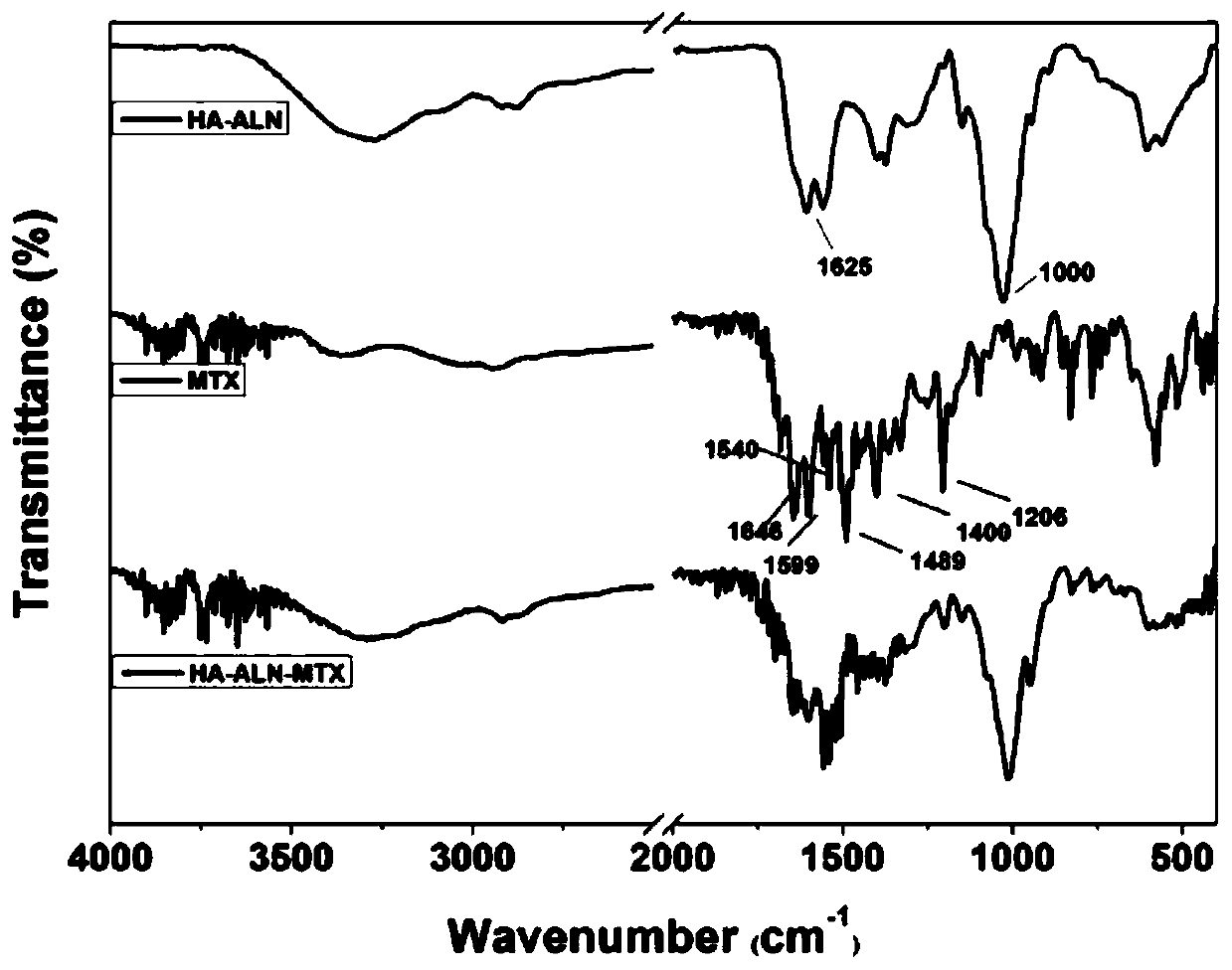
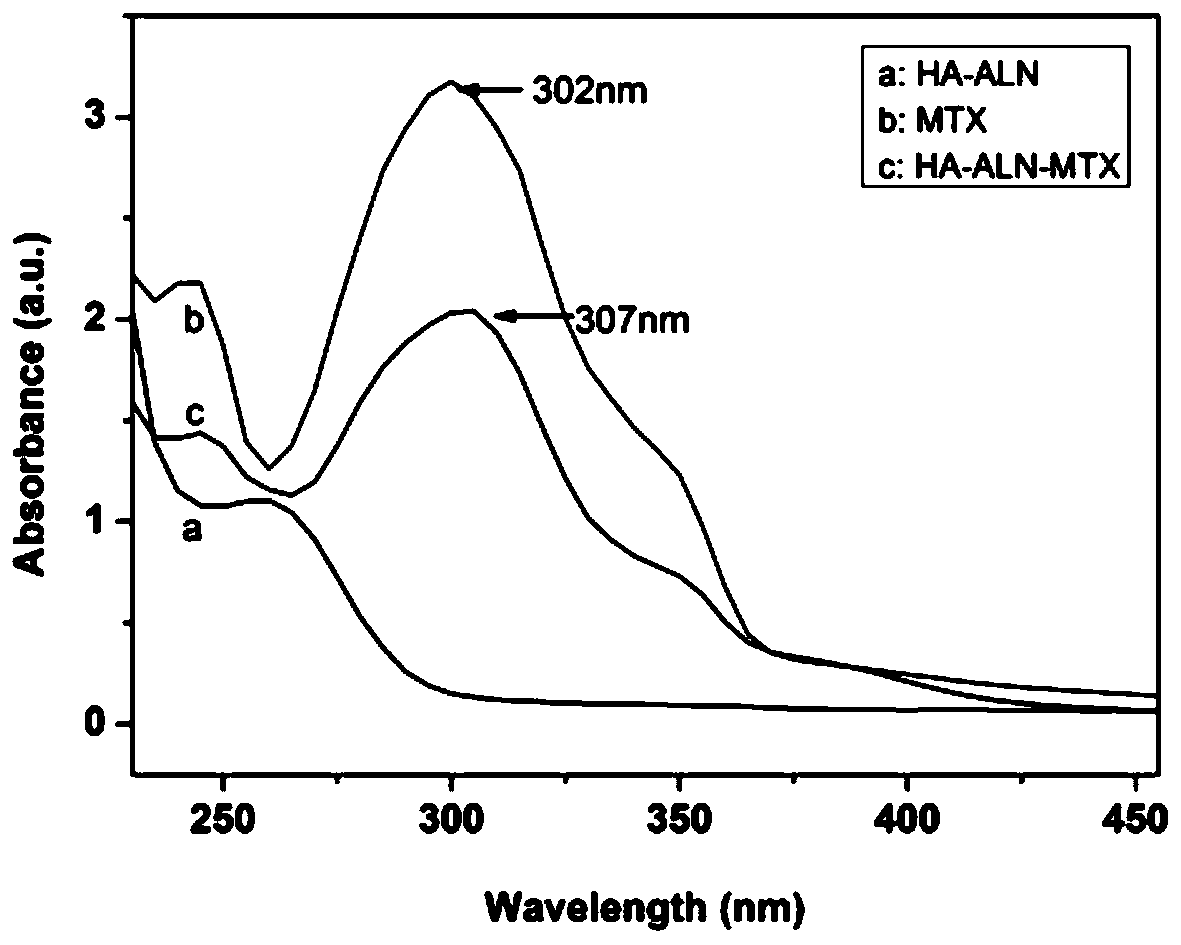
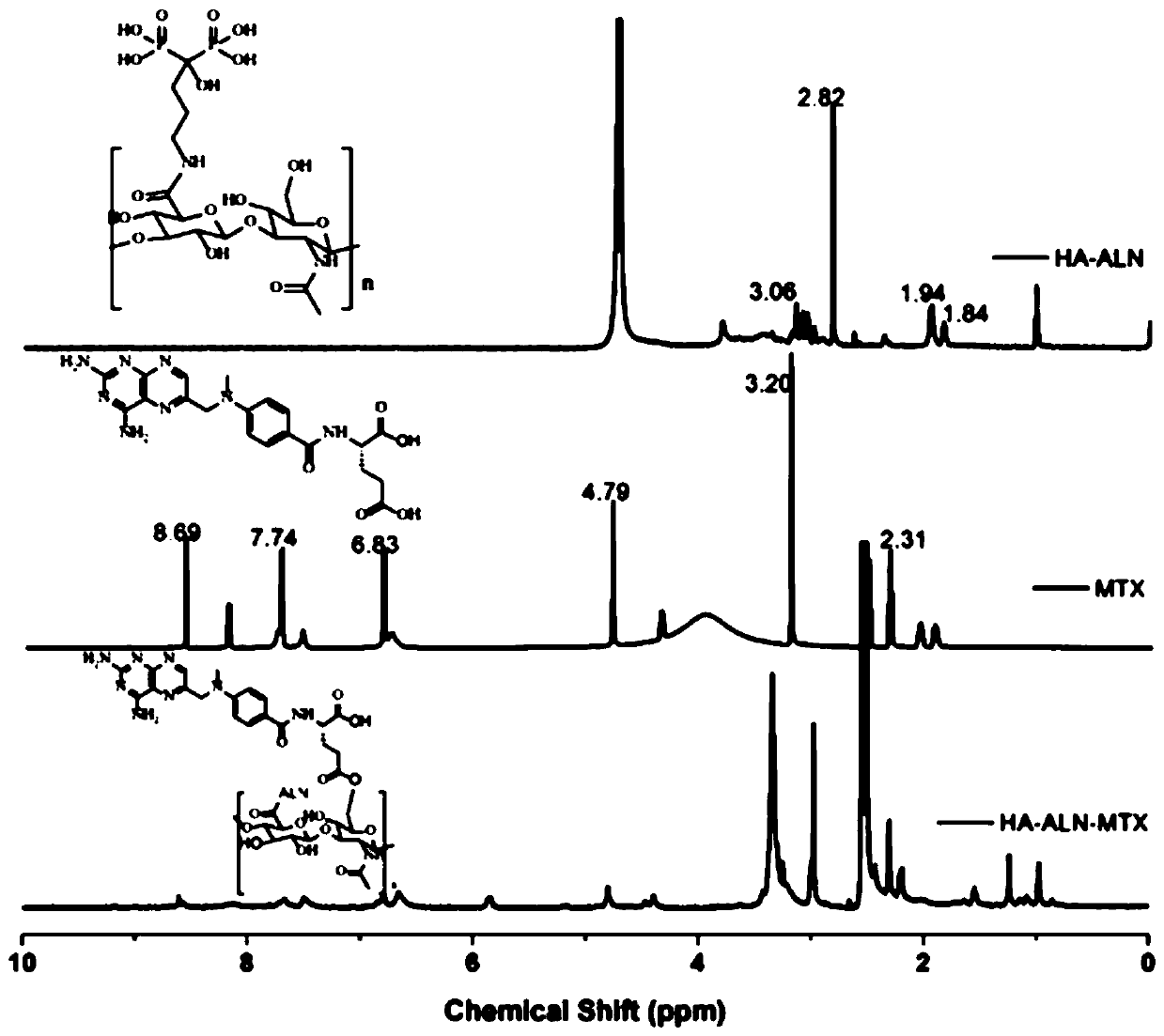
Preparation method of hyaluronic acid-alendronate sodium- methotrexate nanometer granules
A technology of sodium alendronate and hyaluronic acid, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve adverse effects on human body treatment, high solvent toxicity, and complicated processes etc. to achieve the effects of low toxicity and side effects, good dispersion, and high targeting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step is to weigh 800mg of high-efficiency catalyst EDC and 400mg of NHS, add 60mL of deionized water to dissolve, then add 200mg of hyaluronic acid, react for 1 hour under stirring conditions to activate the carboxylic acid part, then add 360mg of alendronate sodium and continue stirring for 24 hours . The obtained product was dialyzed with purified water, the molecular weight of the dialysis bag was 300-1000, stopped after 2 days, and then freeze-dried to collect HA-ALN samples.
[0031] In the second step, the prepared HA-ALN (84mg) was dissolved in 30mL DMF, stirred continuously, then diluted with 25mL DMSO, and stirred.
[0032] In the third step, MTX (84 mg, 0.184 mM) was dissolved in 5 mL DMSO, 152 mg EDC and 90 mg DMAP were weighed, mixed with MTX, and stirred slowly for 30 min under magnetic stirring conditions.
[0033] The fourth step is to mix the solutions of step 2 and step 3, react at room temperature for 24h, and dialyze it for 48h. The molecul...
Embodiment 2
[0037]In the first step, weigh 800mg of high-efficiency catalyst EDC and 400mg of NHS, add 60mL of deionized water to dissolve, then add 200mg of hyaluronic acid, react for 1 hour under stirring to activate the carboxylic acid part, then add 400mg of alendronate sodium and continue stirring for 24 hours . The obtained product was dialyzed with purified water, the molecular weight of the dialysis bag was 300-1000, stopped after 2 days, and then freeze-dried to collect HA-ALN samples.
[0038] In the second step, the prepared HA-ALN (100mg) was dissolved in 30mL DMF, stirred continuously, then diluted with 25mL DMSO, and stirred.
[0039] In the third step, MTX (84 mg) was dissolved in 5 mL DMSO, 152 mg EDC and 90 mg DMAP were weighed, mixed with MTX, and stirred slowly for 30 min under magnetic stirring conditions.
[0040] The fourth step is to mix the solutions of step 2 and step 3, react at room temperature for 24h, and dialyze it for 48h. The molecular weight of the dialys...
Embodiment 3
[0049] In the first step, weigh 800mg of high-efficiency catalyst EDC and 400mg of NHS, add 60mL of deionized water to dissolve, then add 200mg of hyaluronic acid, react for 1 hour under stirring to activate the carboxylic acid part, then add 440mg of alendronate sodium and continue stirring for 24 hours . The obtained product was dialyzed with purified water, the molecular weight of the dialysis bag was 300-1000, stopped after 2 days, and then freeze-dried to collect HA-ALN samples.
[0050] In the second step, the prepared HA-ALN (126mg) was dissolved in 30mL DMF, stirred continuously, then diluted with 25mL DMSO, and stirred.
[0051] In the third step, MTX (84 mg) was dissolved in 5 mL DMSO, 152 mg EDC and 90 mg DMAP were weighed, mixed with MTX, and stirred slowly for 30 min under magnetic stirring conditions.
[0052] The fourth step is to mix the solutions of step 2 and step 3, react at room temperature for 24h, and dialyze it for 48h. The molecular weight of the dialy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com