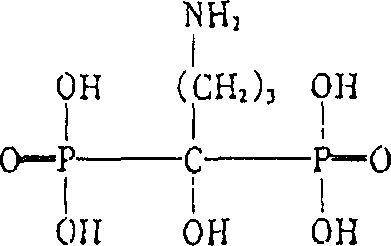
Compound injection contg. alendronate sodium and vitamin D3
An alendronate sodium and injection technology, which is applied in the field of compound injection preparations containing alendronate sodium and vitamin D3, can solve the problem of no alendronate sodium and vitamin D3, etc., and achieve fast absorption and high absorption rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Prepare alendronate sodium vitamin D3 freeze-dried powder injection according to the following formula.
[0055] Specific formula:
[0056] Alendronate Sodium 70g
[0057] Vitamin D370mg (1400IU)
[0058] Hydroxypropyl-β-cyclodextrin 8g
[0059] Mannitol 100g
[0060] Absolute ethanol 10ml
[0061] Water for injection to 2500ml
[0062] A total of 1000 bottles of freeze-dried products were produced
[0063] Specific steps are as follows:
[0064] Preparation of liquid medicine:
[0065] (1) Weigh vitamin D3 according to the prescribed amount and dissolve it in 10ml of absolute ethanol for later use. Weigh hydroxypropyl-β-cyclodextrin according to the prescription, add 450ml of water for injection, stir fully, add the prepared vitamin D3 ethanol solution after it is completely dissolved, stir well and ultrasonicate for 15 minutes to make vitamin D3-cyclodextrin fine solution.
[0066] (2) Weigh alendronate sodium according to the prescription, add 2000ml of wat...
Embodiment 2
[0076] Prepare alendronate sodium vitamin D3 freeze-dried powder injection according to the following formula.
[0077] Specific formula:
[0078] Alendronate Sodium 35g
[0079] Vitamin D335mg (1400IU)
[0080] Hydroxypropyl-β-cyclodextrin 5g
[0081] Dextran 50g
[0082] Absolute ethanol 5ml
[0083] Water for injection to 2000ml
[0084] A total of 1000 bottles of freeze-dried products were produced
[0085] Specific steps are as follows:
[0086] Preparation of liquid medicine:
[0087] (1) Weigh vitamin D3 according to the prescribed amount and dissolve it in 5ml of absolute ethanol for later use. Weigh hydroxypropyl-β-cyclodextrin according to the prescription, add 300ml of water for injection, stir thoroughly, add the prepared vitamin D3 ethanol solution after it is completely dissolved, stir well and then ultrasonicate for 15 minutes to make vitamin D3-cyclodextrin fine solution.
[0088] (2) Weigh alendronate sodium according to the prescription, add 1500ml ...
Embodiment 3
[0096] Prepare alendronate sodium vitamin D3 injection according to the following formula.
[0097] Specific formula:
[0098] Alendronate Sodium 35g
[0099] Vitamin D335mg (1400IU)
[0100] Hydroxypropyl-β-cyclodextrin 5g
[0101] Absolute ethanol 5ml
[0102] Water for injection to 2000ml
[0103] A total of 1000 injections were prepared
[0104] Specific steps are as follows:
[0105] Preparation of liquid medicine:
[0106] (1) Weigh vitamin D3 according to the prescribed amount and dissolve it in 5ml of absolute ethanol for later use. Weigh hydroxypropyl-β-cyclodextrin according to the prescription, add 300ml of water for injection, stir thoroughly, add the prepared vitamin D3 ethanol solution after it is completely dissolved, stir well and then ultrasonicate for 15 minutes to make vitamin D3-cyclodextrin fine solution.
[0107] (2) Weigh alendronate sodium according to the prescription, add 1500ml of water for injection, and stir thoroughly at 70°C for 30 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com