Prenylflavonoids from jackfruit and their new use in anti-osteoporosis
A technology for prenyl flavonoids and osteoporosis, which is applied in the field of medicine and can solve problems such as the application of osteoporosis that has not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of isopentenyl flavonoids in jackfruit
[0020] Take jackfruit root medicinal material (17Kg), extract with 95% ethanol leakage, and concentrate the extract under reduced pressure to obtain 1.5Kg of extract. The extract was suspended in 2 L of water, extracted with petroleum ether, chloroform, ethyl acetate and n-butanol in sequence (volume ratio 1:1), and concentrated to dryness respectively. Take 532g of the extract from the chloroform extraction part, mix the sample of HP-20 macroporous adsorption resin (weight ratio 1:1), and put it on a HP-20 macroporous adsorption resin column (column size: 15*55cm, 5.3Kg), and mix with ethanol-water Gradient elution (0-95%) followed by a final rinse with acetone. Take 60% ethanol elution fractions, and perform ODS reverse-phase silica gel column chromatography with ethanol-water (2:5, 9:10) to obtain 6 fractions: A-F. Fraction C was subjected to MCI column chromatography with ethanol-water (3:5, 9:10) to o...
Embodiment 2
[0023] Example 2 Structure identification of isopentenyl flavonoids in jackfruit
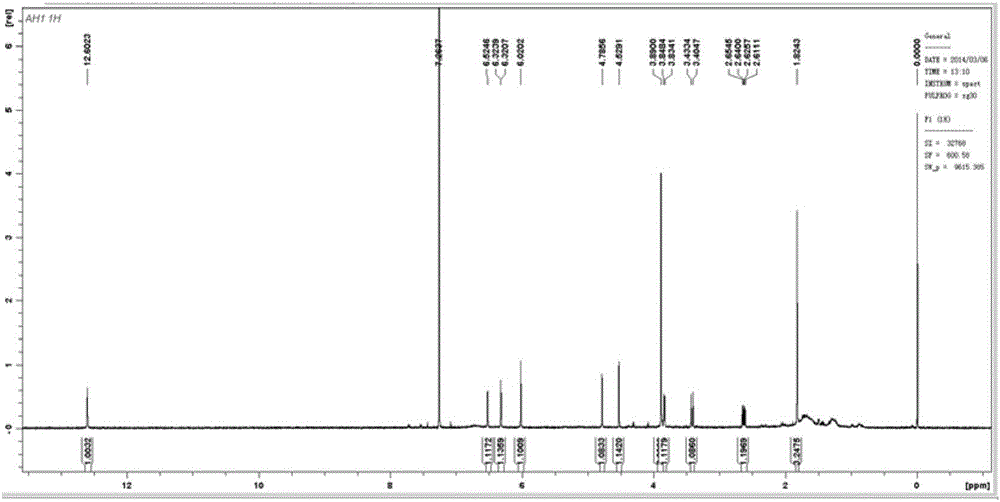
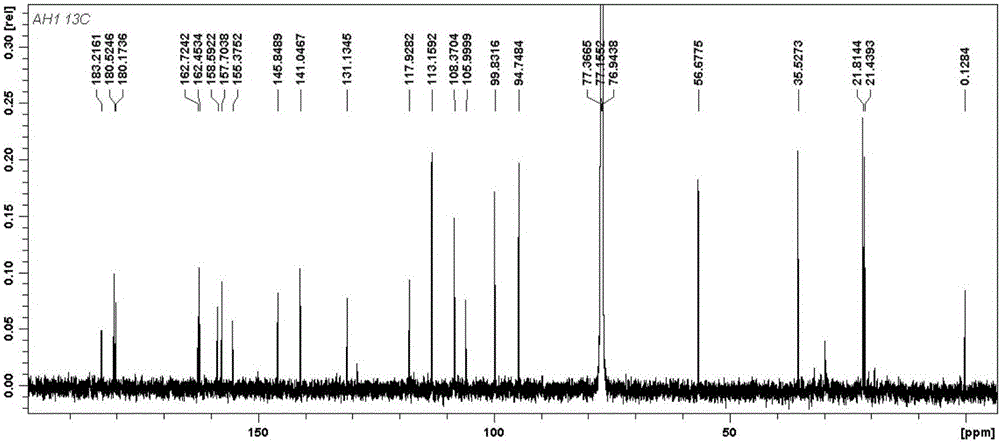
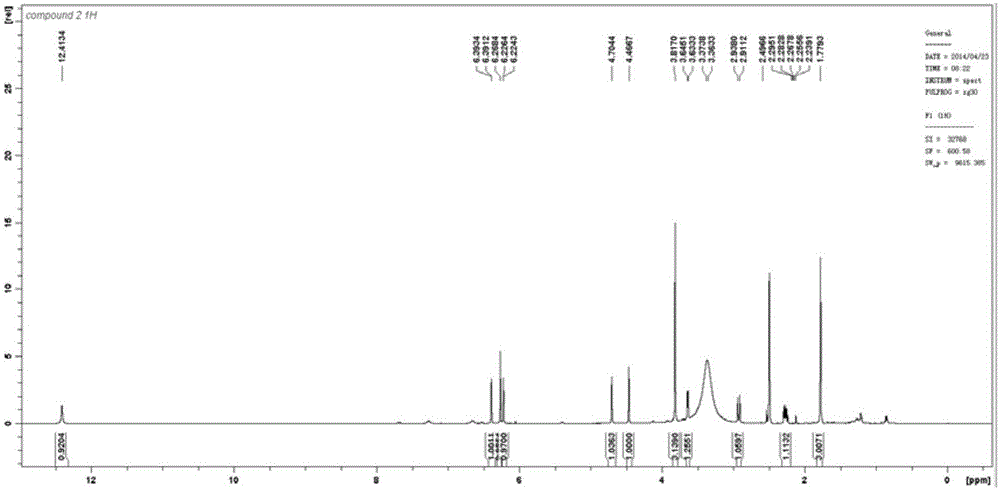
[0024] The isolated monomers were identified as seven prenylated flavonoids by high resolution mass spectrometry (HR-ESI-MS) and nuclear magnetic resonance spectroscopy (1D NMR and 2D NMR). Among them, compounds 1-5 are known compounds, while compounds 6 and 7 are two new compounds that have not been reported in the literature.
[0025] Among them, compound 1 was identified as 5′-hydroxycudraflavone A. C 25 H 22 O 7 , yellow amorphous powder. 1 HNMR(CD 3 OD, 600MHz) δppm 7.15 (1H, s, H-6'), 6.65 (1H, d, J=10.0Hz, H-16), 6.39 (1H, s, H-H-3'), 6.35 (1H, s , H-8), 6.10 (1H, d, J=9.0Hz, H-11), 5.69 (1H, d, J=10.0Hz, H-17), 5.44 (1H, d, J=9.0Hz, H -12), 1.97 (3H, s, H-15), 1.70 (3H, s, H-14), 1.47 (6H, s, H-19, 20); 13 CNMR(CD 3 OD, 150MHz) δppm: 157.7(C-2), 110.5(C-3), 179.6(C-4), 157.8(C-5), 106.5(C-6), 160.3(C-7), 96.0( C-8), 157.3(C-9), 106.3(C-10), 70.2(C-11), 122.3(C-12), 139.7(C-13),...
Embodiment 3
[0034] Example 3 Experiment on the inhibitory activity of isopentenyl flavonoids in jackfruit on CatK
[0035] 40 [mu]L of CatK (diluted with 0.1% Brii35) was added to the 96-well plate. Then 35 μL of buffer solution containing the test compound (containing 400 mM NaH) was added 2 PO 4-Na 2 HPO 4 buffer, 88 mM DTT and 4 mM EDTA, pH 6.8), incubated at 25° for 10 minutes to allow sufficient binding of the compound to the enzyme. 25 μL of the substrate Z-GPR-AMC (diluted in 1% DMSO) was then added to the mixture to start the reaction. The final concentrations of substrate and CatK were 5 μM and 5.6 μM, respectively, with a total volume of 100 μL per well. After the reaction system was incubated at 37 °C for 2 h, 50 μL of reaction stop solution (containing 100 mM CH) was added. 3 COOH-CH 3 COONa, 100mMCl 3 CCOONa, pH 4.3) into each well. The fluorescence signal intensity of AMC in the reaction system was detected with an Omega microwell spectrophotometer (BMG LABTECH, Ger...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com