Serpin and minimum functional fragment and immunosuppression effect thereof
A serine protease and functional fragment technology, applied in the field of biomedicine, can solve the problems of lack of vaccines for ticks and tick-borne diseases, lack of safe and efficient repellent drugs, etc., and achieve the effect of easy absorption, small molecular weight, and poor immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
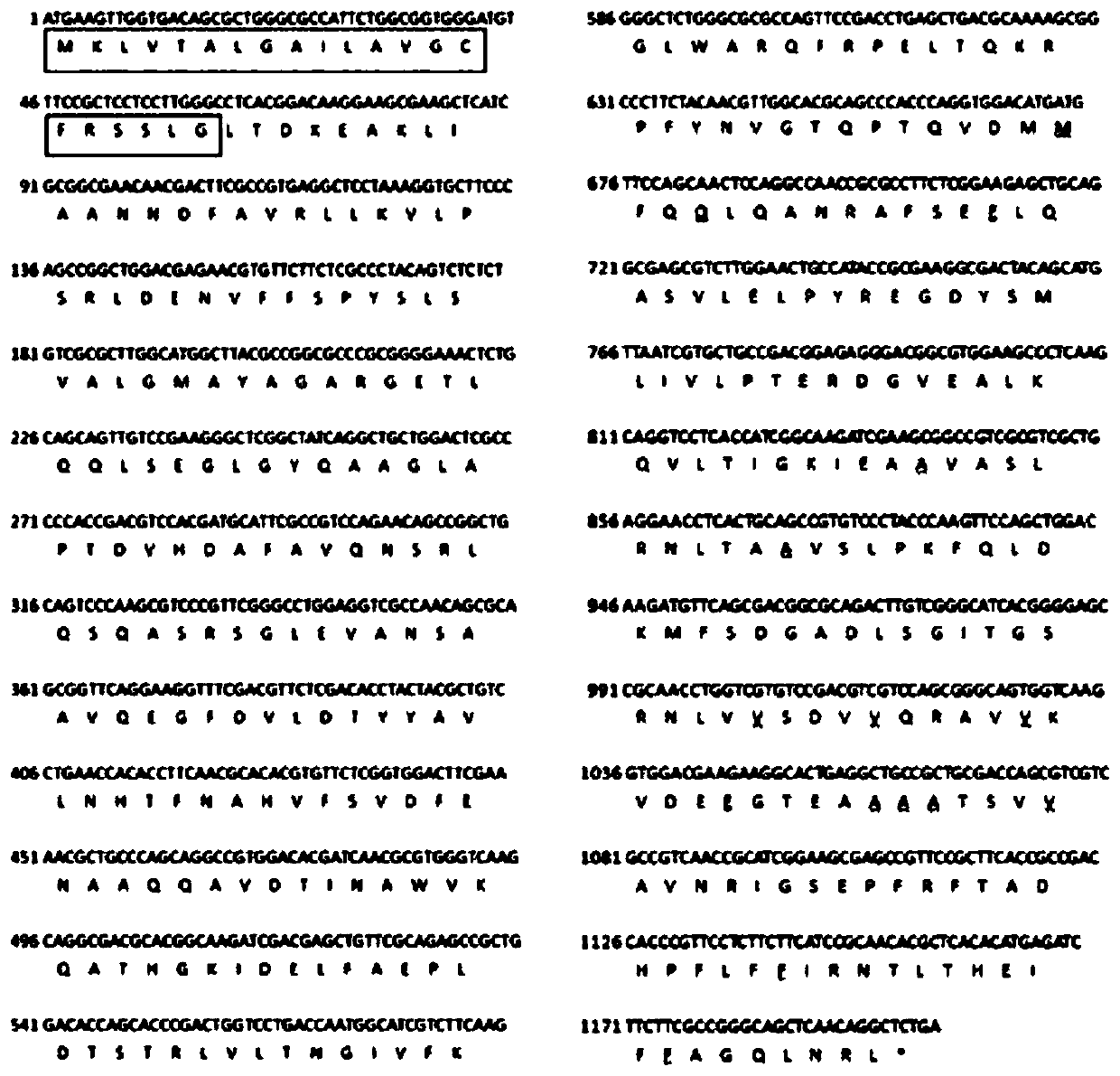
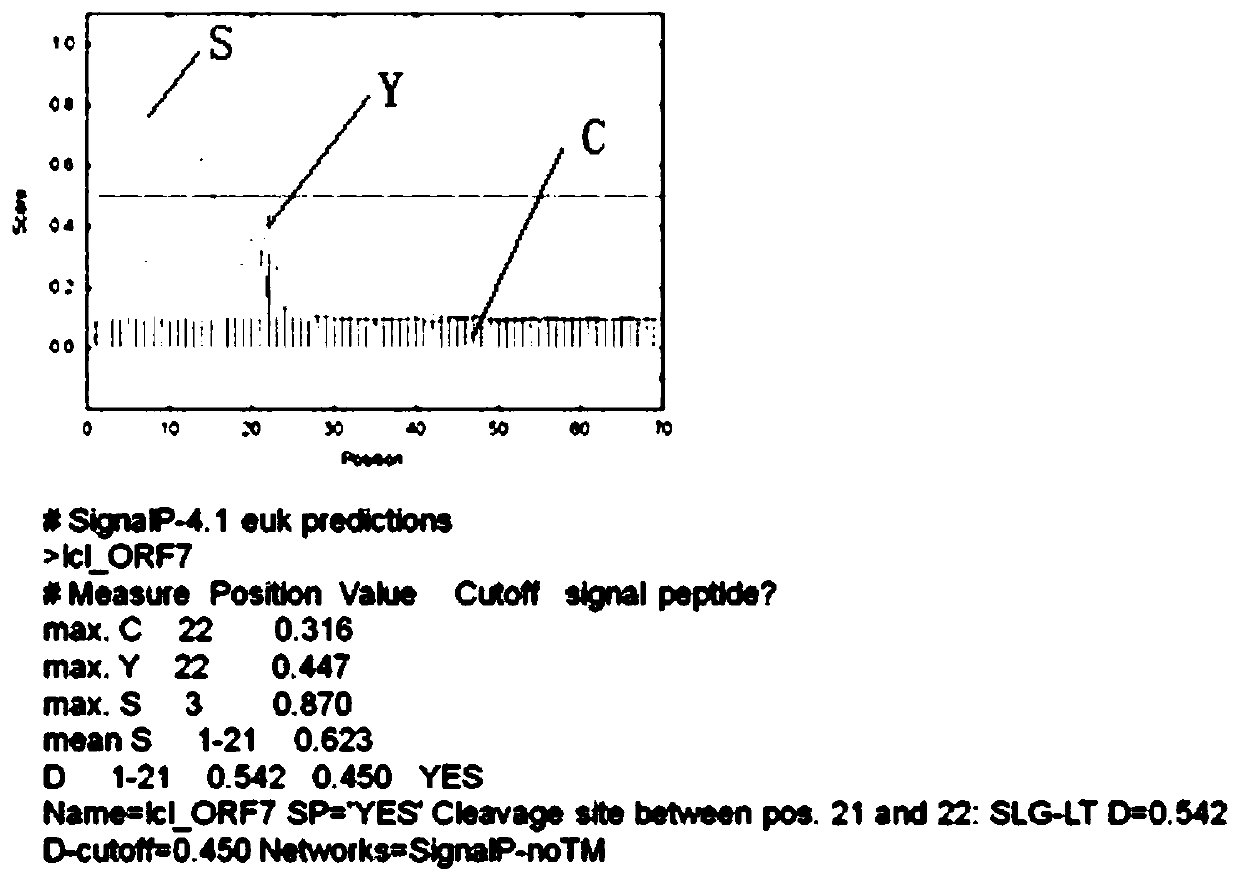
[0042] Provide a kind of serine protease inhibitor SA, its protein expression process is:
[0043] (1) Clone the SA gene into the prokaryotic expression vector pGEX-6P-2
[0044] Extract the RNA of the longhorned blood tick, prepare cDNA, amplify the protein coding region fragment of the target gene from the cDNA library through gene-specific primers, and connect the coding region of the target gene to the Escherichia coli expression vector pGEX-6P- 2 in.
[0045] (2) Recombinant expression of serine protease inhibitor SA in Escherichia coli
[0046] The constructed recombinant pGEX-6P-2 target gene expression plasmid was transformed into Escherichia coli BL21 strain, and IPTG was used to induce the expression of the recombinant target protein.
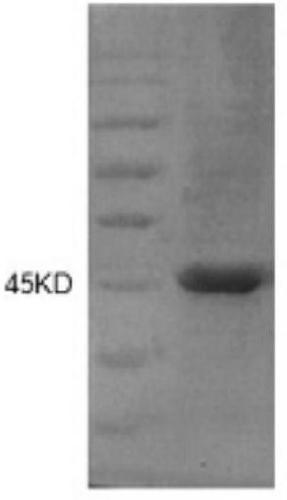
[0047] (3) The method of column chromatography purifies recombinant serine protease inhibitor SA protein
[0048] Use GST medium to purify the recombinant target protein, and use specific protease to digest on the column to remove ...
Embodiment 2
[0051] Activity Inhibition of Serine Protease Inhibitor SA
[0052] 1. The identification experiment of serine protease inhibitor SA on the active inhibitory effect of cathepsin G (cathepsin G) and blood coagulation factor X (FXa), including steps:
[0053] (a) The total reaction system is 200ul, Cathepsin G (6.6nM) and FXa (0.33nM) were incubated with SA (0.1uM, 0.4uM) at room temperature for 30min.
[0054] (b) Add substrates at a final concentration of 250uM: Cathepsin G, methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide; FXa, chromogenic substrate S2765. 37°C, total reaction 90min, use enzyme labeling every 30min Instrument measurement, A = 405nm.
[0055] Such as Figure 4 and Figure 5 As shown, it was found that serine protease inhibitor SA inhibited the enzymatic activity of Cathepsin G and FXa in a dose-dependent manner.
[0056] 2. Identification experiment of the inhibitory effect of serine protease inhibitor SA on the activity of cathepsin B (cathepsin B)
[0057...
Embodiment 3
[0074] The effect of the serine protease inhibitor SA on the secretion of inflammatory factors by BMDMs or BMDCs stimulated by bacterial endotoxin lipopolysaccharide LPS
[0075] Stimulate BMDMs or BMDCs with bacterial endotoxin lipopolysaccharide (LPS), and detect the effect of SA on the expression of inflammatory factors at the mRNA and protein levels by real-time fluorescent quantitative PCR and enzyme-linked immunosorbent assay (ELISA) experiments.
[0076] Sample preparation and cell experiment steps are as follows:
[0077](a) BMDMs or BMDCs cells were plated in a 24-well plate, and three groups were set up: NC (blank control), PBS (negative control), and SA.
[0078] (b) PBS and endotoxin-depleted SA were incubated with cells for two hours.
[0079] (c) Cells in PBS and SA groups were stimulated with 1 μg / ml bacterial lipopolysaccharide (LPS).
[0080] (d) After being stimulated for 12 hours, cellular RNA was extracted and reverse-transcribed into cDNA, which was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com