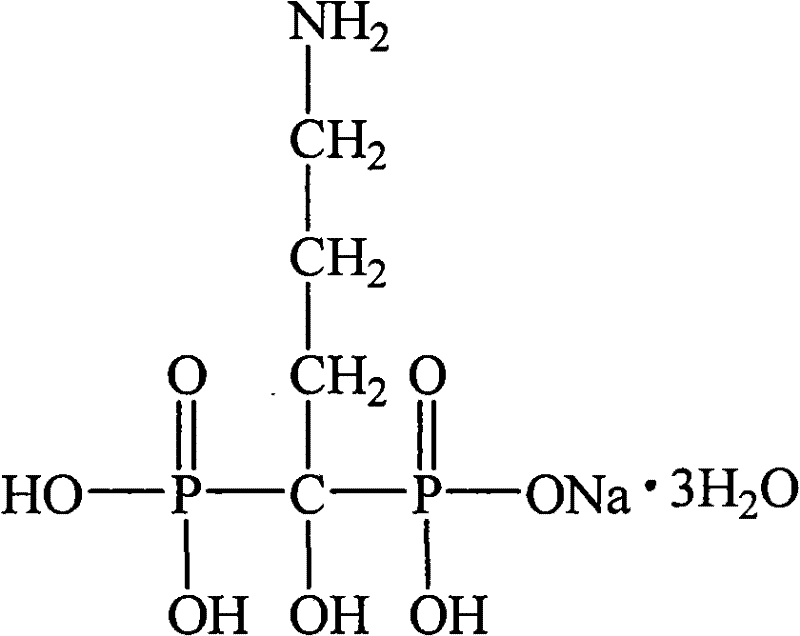
Alendronate sodium intestine-sol capsule and preparation method thereof
A technology of alendronate sodium and enteric-coated capsules, applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of hindering osteoporosis patients and inconvenient taking, and achieve good economic and Social benefits, simplifying the production process, and ensuring the effect of drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
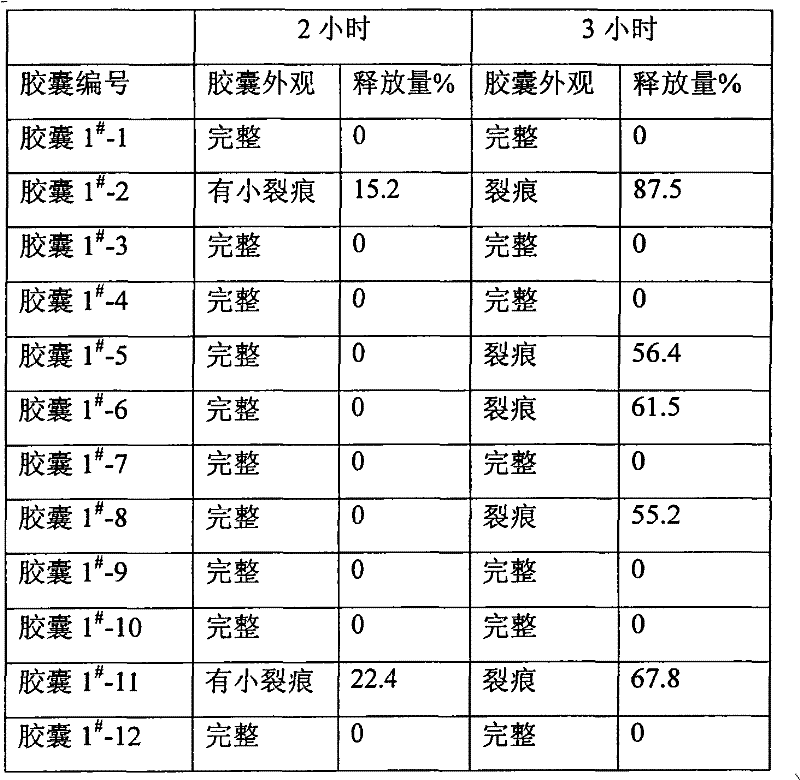
[0028] Example 1 Enteric-coated capsules of alendronate sodium without adding protective agent, hereinafter referred to as capsule 1 #
[0029] The prescription is as follows (1000 capsules):
[0030] Alendronate Sodium 10g
[0031] Starch 80g
[0032] 2% HPMC solution (prepared with 50% ethanol) 30ml
[0033] Magnesium stearate 0.5g
[0034] Preparation process: Alendronate Sodium is passed through a 100-mesh sieve, starch and magnesium stearate are passed through an 80-mesh sieve, and the Alendronate Sodium and starch of the formula quantity are fully mixed; with 2% HPMC solution (with 50% ethanol Preparation) Wet granulation, passing through a 24-mesh sieve to obtain wet granules, drying at 55°C to obtain dry granules; sizing the dry granules through a 20-mesh sieve, adding magnesium stearate, mixing evenly, and filling into enteric-coated capsules , to obtain alendronate sodium enteric-coated capsules.
Embodiment 2
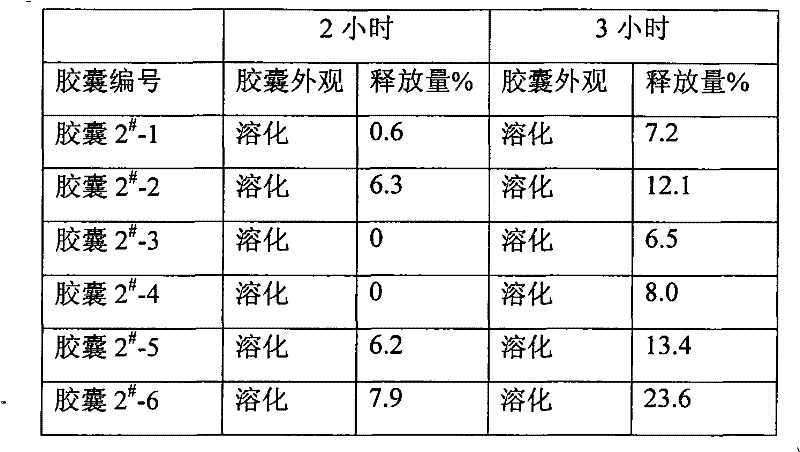
[0035] Example 2 Enteric-coated capsules of alendronate sodium using common technology, hereinafter referred to as capsule 2 #
[0036] The prescription is as follows (1000 capsules):
[0037] Alendronate Sodium 10g
[0038] Microcrystalline Cellulose 80g
[0039] 2% HPMC solution (prepared with 50% ethanol) 30ml
[0040] Acrylic II 9g
[0041] Magnesium stearate 0.5g
[0042] Preparation process: Alendronate sodium is passed through a 100-mesh sieve, microcrystalline cellulose and magnesium stearate are passed through a 80-mesh sieve, and the alendronate sodium and microcrystalline cellulose in the prescribed amount are fully mixed; The solution (prepared with 50% ethanol) is wet granulated, passed through a 24-mesh sieve to obtain wet granules, dried at 55°C to obtain dry granules; coated in a granule coating machine, and the enteric coating solution is polypropylene resin II (made into an aqueous solution with a mass percent concentration of 5%), uniformly sprayed on ...
Embodiment 3
[0043] Embodiment 3 Alendronate sodium enteric-coated capsules of the present invention, hereinafter referred to as capsule 3 #
[0044] The prescription is as follows (1000 capsules):
[0045] Alendronate Sodium 10g
[0046] Starch 80g
[0047] Carbomer 934P 1g
[0048] 2% HPMC solution (prepared with 50% ethanol) 30ml
[0049] Magnesium stearate 0.5g
[0050] Preparation process: pass alendronate sodium through a 100-mesh sieve, starch, carbomer 934P, and magnesium stearate through a 80-mesh sieve, take the formula amount of alendronate sodium and starch and mix well; use 2% HPMC solution (Prepared with 50% ethanol) Wet granulation, passing through a 24-mesh sieve to obtain wet granules, drying at 55°C to obtain dry granules; sizing the dry granules with 20-mesh sieve, adding Carbomer 934P and magnesium stearate , mixed evenly, filled into enteric-coated capsules to obtain alendronate sodium enteric-coated capsules
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com