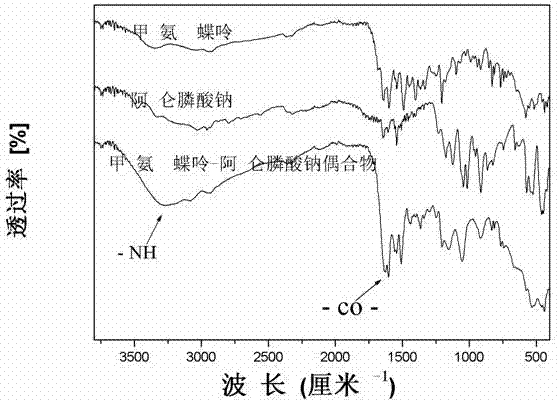
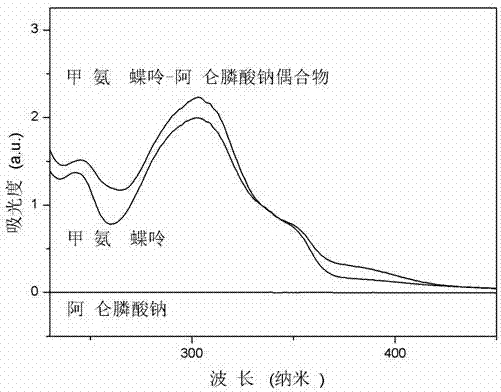
Preparation method of methotrexate and alendronate sodium conjugate
A technology of sodium alendronate and methotrexate, applied in chemical instruments and methods, medical preparations of non-active ingredients, drug combinations, etc., can solve problems such as complicated process production, cumbersome operation requirements, and inability to produce on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
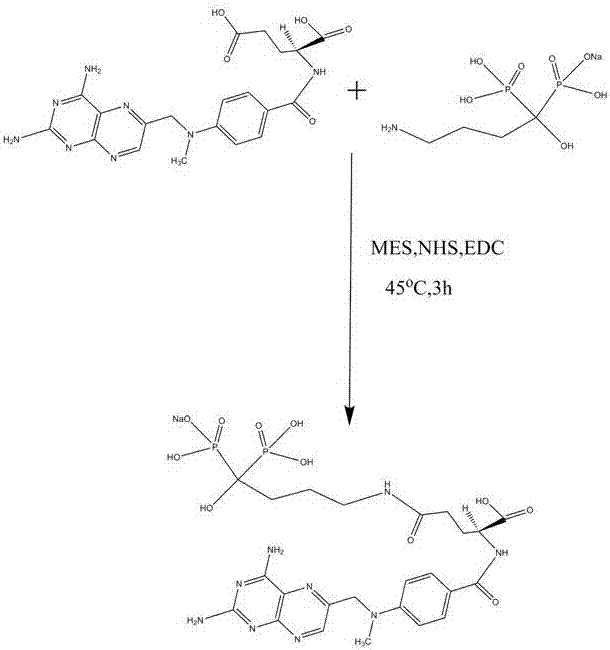
[0033] Weigh 3.6 g of methotrexate with 90 ml of ultrapure water and mix it with an electronic balance, add dropwise acetone until the methotrexate is basically dissolved to form a methotrexate solution.
[0034] To the methotrexate solution, add 3 g of 2-morpholineethanesulfonic acid (MES), 1.8 g of N-hydroxysuccinimide (NHS), 3 g of 1-(3-dimethylamino)-3- Ethylcarbodiimide hydrochloride (EDC).
[0035] Then add 6 g of alendronate sodium, place it on a magnetic stirrer, and continue to stir and react for 3 hours.
[0036] After the reaction, the obtained solution was poured into a 300 molecular weight dialysis bag, and placed in a large beaker filled with ultrapure water for dialysis for 24 h, and the ultrapure water was changed every 2-6 h.
[0037] After the dialysis is finished, take out the material in the dialysis bag, freeze the taken out material in an open container and wrap it with a plastic wrap, then punch and dry for 48 hours to obtain the desired target product....
example 2
[0039] Weigh 7.2g of methotrexate with an electronic balance and mix it with 180ml of ultrapure water, add dropwise chloroform until the methotrexate is basically dissolved to form a methotrexate solution.
[0040] Add 6 g of 2-morpholineethanesulfonic acid (MES), 3.6 g of N-hydroxysuccinimide (NHS), 6 g of 1-(3-dimethylamino)-3 to the forming methotrexate solution - Ethylcarbodiimide hydrochloride (EDC).
[0041] Then add 12 g of alendronate sodium, place it on a magnetic stirrer, and continue to stir and react for 12 hours.
[0042] After the reaction, the obtained solution was poured into a 600 molecular weight dialysis bag, and placed in a large beaker filled with ultrapure water for dialysis for 24 h, and the ultrapure water was changed every 2-6 h.
[0043] After the dialysis is finished, take out the material in the dialysis bag, freeze the taken out material in an open container and wrap it with a plastic wrap, then punch and dry for 72 hours to obtain the desired tar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com