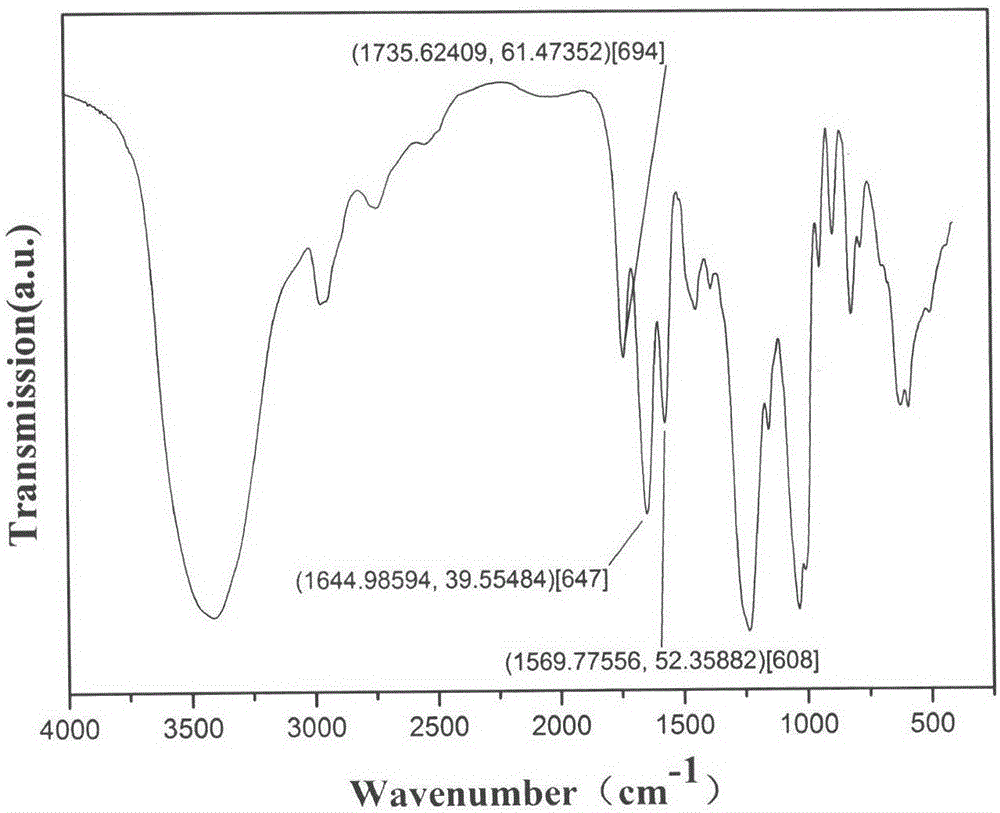
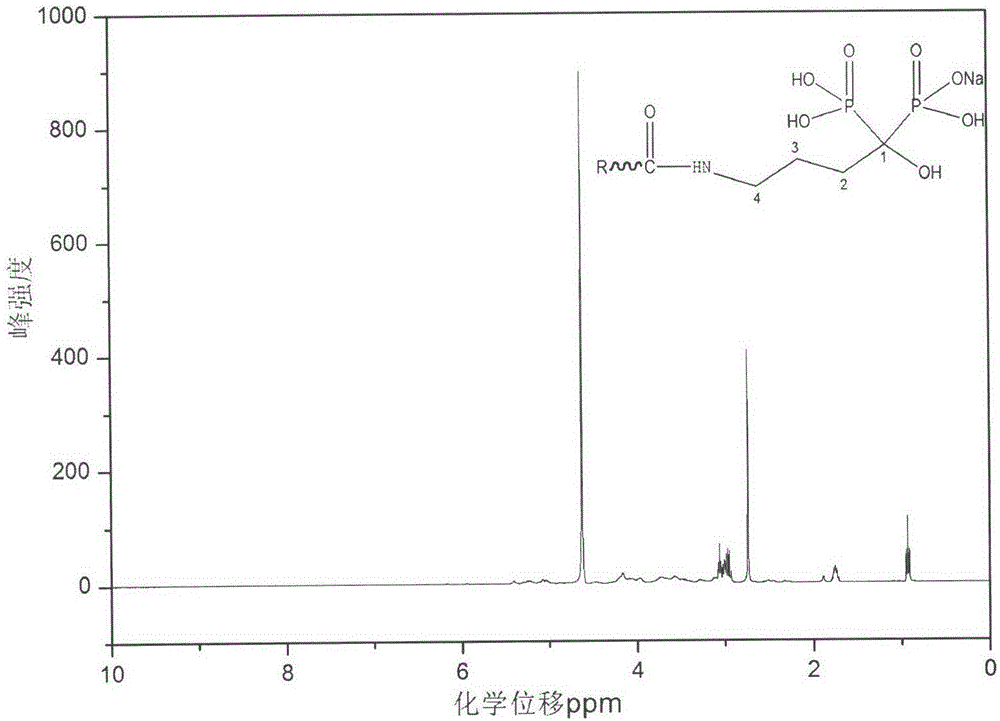
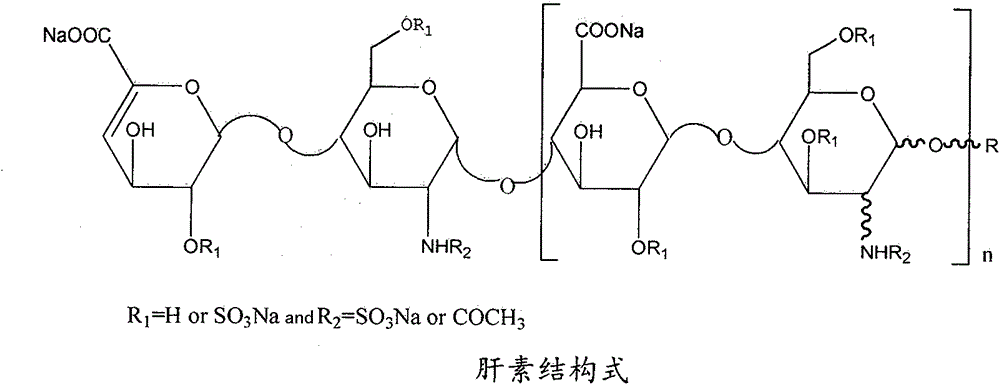
Heparin bisphosphonate derivative and synthetic method and application thereof
A technology of bisphosphonates and a synthesis method, which can be used in drug combinations, bone diseases and other directions, can solve problems such as inability to mass-produce, high preparation costs, and complex processes, and achieves improved rehabilitation treatment levels, simple preparation methods, and low process requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of heparin alendronate sodium derivatives
[0041] 1. Dissolve 1g of heparin in 20ml of ultrapure water;
[0042] 2. Add 1.2331g of 2-morpholineethanesulfonic acid (EMS), 0.7269g of N-hydroxysuccinimide (HOSU), 1.2165g of 1-(3-dimethylamino)-3-ethyl carbon Diimine hydrochloride (EDC) was dissolved in 30ml of ultrapure water;
[0043] 3. Dissolve 2.0534g of alendronate sodium in 100ml of ultrapure water;
[0044] 4. Add the solution obtained in (2) into the solution of (1), place it on a magnetic stirrer, and stir for activation for 5 hours;
[0045] 5. After 5 hours, add the solution obtained in (3) into the solution obtained in (4), and stir for 24 hours;
[0046] 6. After 24 hours, pour the solution obtained in (5) into a dialysis bag, dialyze in ultrapure water for 48 hours, and change the water every 4 hours;
[0047] 7. After 48 hours, freeze-dry the solution in the dialysis bag in (6) to obtain the desired target product.
[0048] 8. Th...
Embodiment 2
[0049] Example 2: Preparation of heparin alendronate sodium derivatives
[0050] (1) Dissolve 53.2mg heparin in 5ml ultrapure water;
[0051] (2) Dissolve 73.6 mg of EMS, 42.3 mg of HoSu, and 61.2 mg of EDC in 15 ml of ultrapure water.
[0052] (3) Dissolve 410.68mg of alendronate sodium in 5ml of ultrapure water
[0053] (4) Add the solution obtained in (2) into the solution of (1), and place it on a magnetic stirrer to stir and activate for 5 hours;
[0054](5) After 5h, the solution obtained in (3) was added to the solution obtained in (4), and the reaction was stirred for 24h;
[0055] (6) After 24 hours, pour the solution obtained in (5) into a dialysis bag, dialyze in ultrapure water for 48 hours, and change the water every 4 hours;
[0056] (7) After 48 hours, freeze-dry the solution in the dialysis bag in (6) to obtain the desired target product.
[0057] The present invention applies the product:
[0058] It is used for preventing and preventing osteolytic lesion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com