Preparation method and application of 2-amino-3,5-bibromobenzyl intermediate compound
A technology of dibromobenzyl and compound, which is applied in the field of medicine and chemical industry, can solve the problems of corrosion and cumbersome handling, and achieve the effects of less environmental pollution, mild reaction conditions, and avoiding genotoxic impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
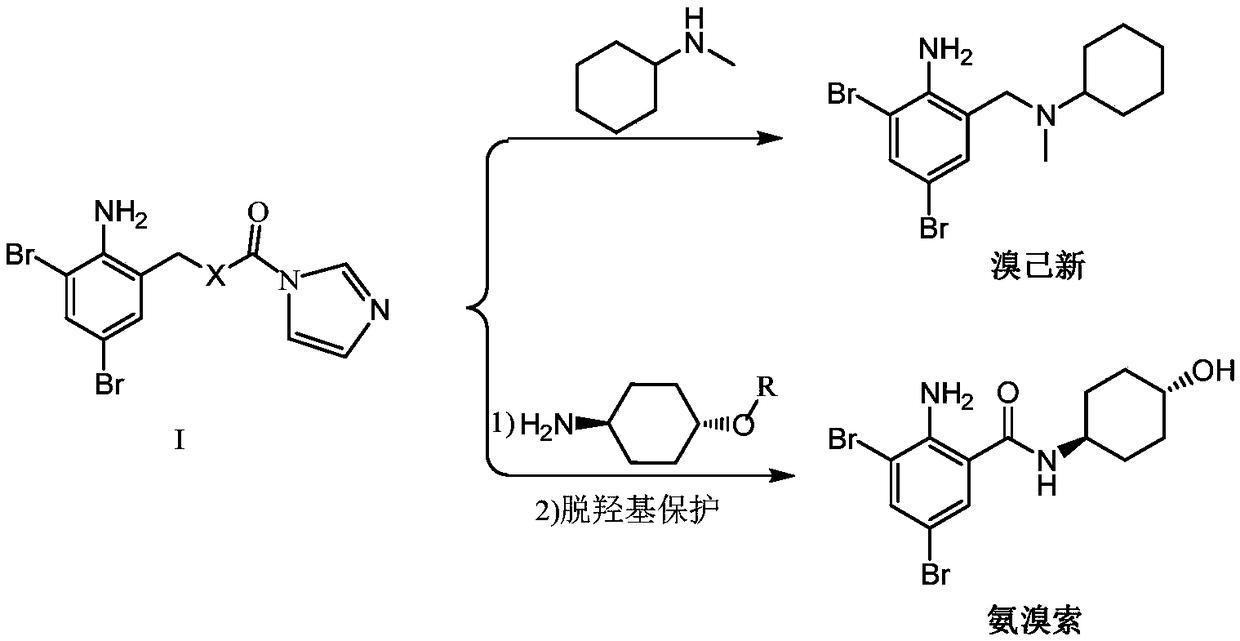
[0049] On the other hand, the present invention further discloses a method for preparing the above-mentioned 2-amino-3,5-dibromobenzyl intermediate compound I, the method comprising the following steps:
[0050]
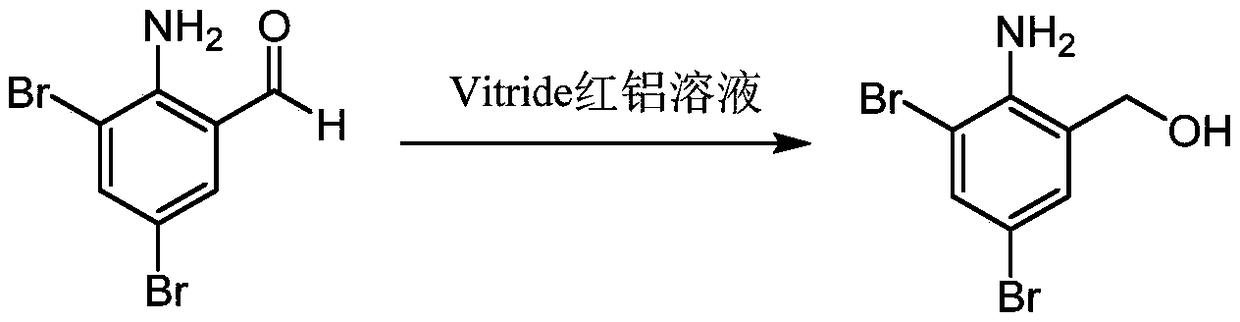
[0051] S1, in the toluene solution of the compound of formula II, reduce the aldehyde group or thioaldehyde group of the compound of formula II with a reducing agent to obtain the compound of formula III;
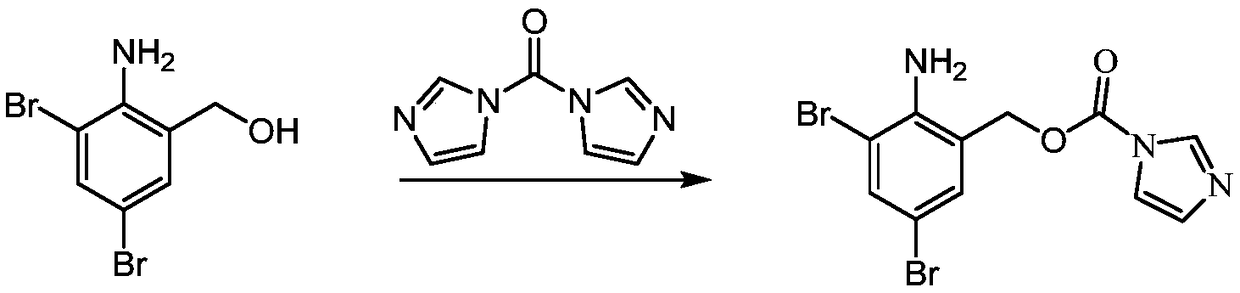
[0052] S2, in the tetrahydrofuran solution of the compound of formula III, react the compound of formula III with the activator of formula IV to obtain compound I;
[0053] The activator of formula IV is N'N-carbonyldiimidazole.
[0054] As a further improvement of the embodiment of the present invention, in the step S1, the reducing agent is selected from at least one of Vitride red aluminum solution, sodium borohydride or potassium borohydride;
[0055] As a further improvement of the embodiment of the present invention, the molar ratio of the compound of fo...
Embodiment 1
[0076] This example discloses a method for preparing 2-amino-3,5-dibromobenzyl alcohol, which specifically includes the following steps:
[0077] In a 500ml three-neck flask, under nitrogen protection, add 20.0g (71.1mmol, 1.0eg) 2-amino-3,5-dibromobenzaldehyde, 200ml toluene, stir to dissolve, cool down to -5°C, and add Vitride red dropwise while keeping warm Aluminum solution 42.6ml (107.5mmol, 1.5eg, specification: 70% toluene solution, 3.6mol / L), the solution changed from light yellow to yellow. After the dropwise addition, return to room temperature for reaction for 5-6h. After the reaction was complete, the temperature was lowered to 10° C., and 20 ml of 10% sodium hydroxide was added dropwise to quench the reaction. Add 100ml of water for extraction and separation, wash the organic phase with pure water and saturated brine, dry the organic phase with anhydrous sodium sulfate, filter, and evaporate the filtrate to give 19.8 g of a yellow solid, which is the product, wit...
Embodiment 2
[0079] This example discloses a method for preparing 2-amino-3,5-dibromobenzyl-1H-imidazole-1-carbonate, which specifically includes the following steps:
[0080] In a 250ml single-necked bottle, add 10.0g (35.6mmol, 1.0eg) 2-amino-3,5-dibromobenzyl alcohol, 60ml tetrahydrofuran and stir to dissolve. Under nitrogen protection, cool down to 5°C, and add 6.34g ( 39.1mmol, 1.1eg) N'N-carbonyldiimidazole, control the temperature to be less than 15°C, after completion, rise to 20°C and react for 1h, TLC plate, the reaction is complete, evaporate most of the tetrahydrofuran solvent under reduced pressure below 30°C to obtain a concentrated solution , adding 100ml of 10% citric acid ice water and 100ml of ethyl acetate for extraction, layered, the organic phase was washed with saturated brine, dried, filtered, and spin-dried at room temperature to obtain 13.0g of white solid, which was the product, with a yield of 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com