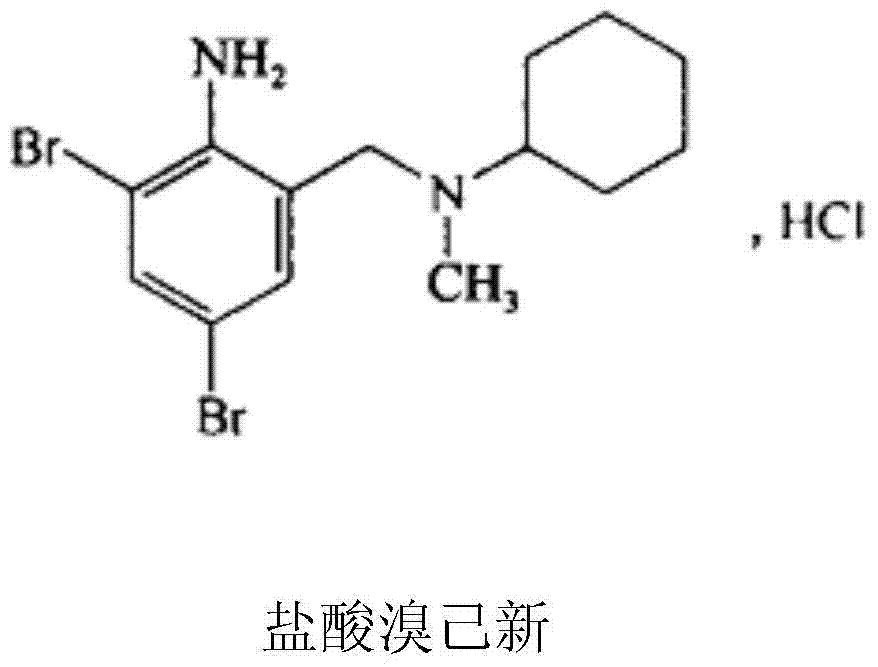
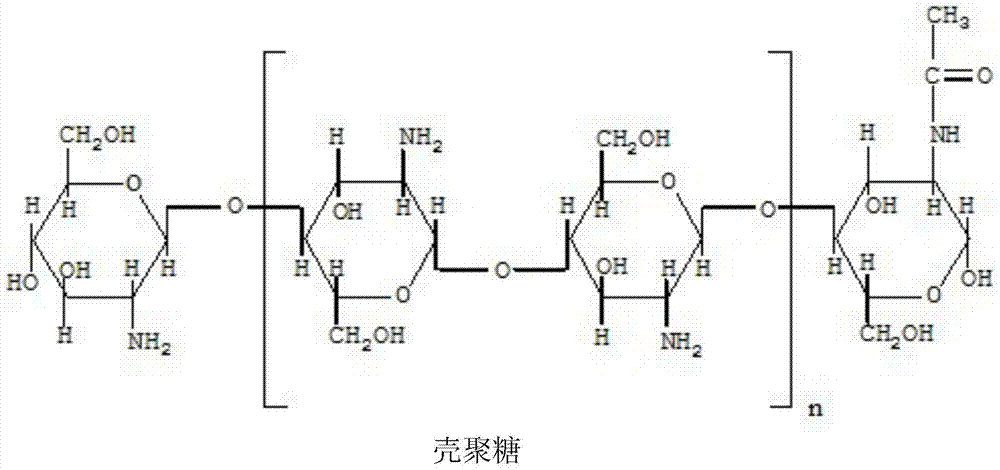
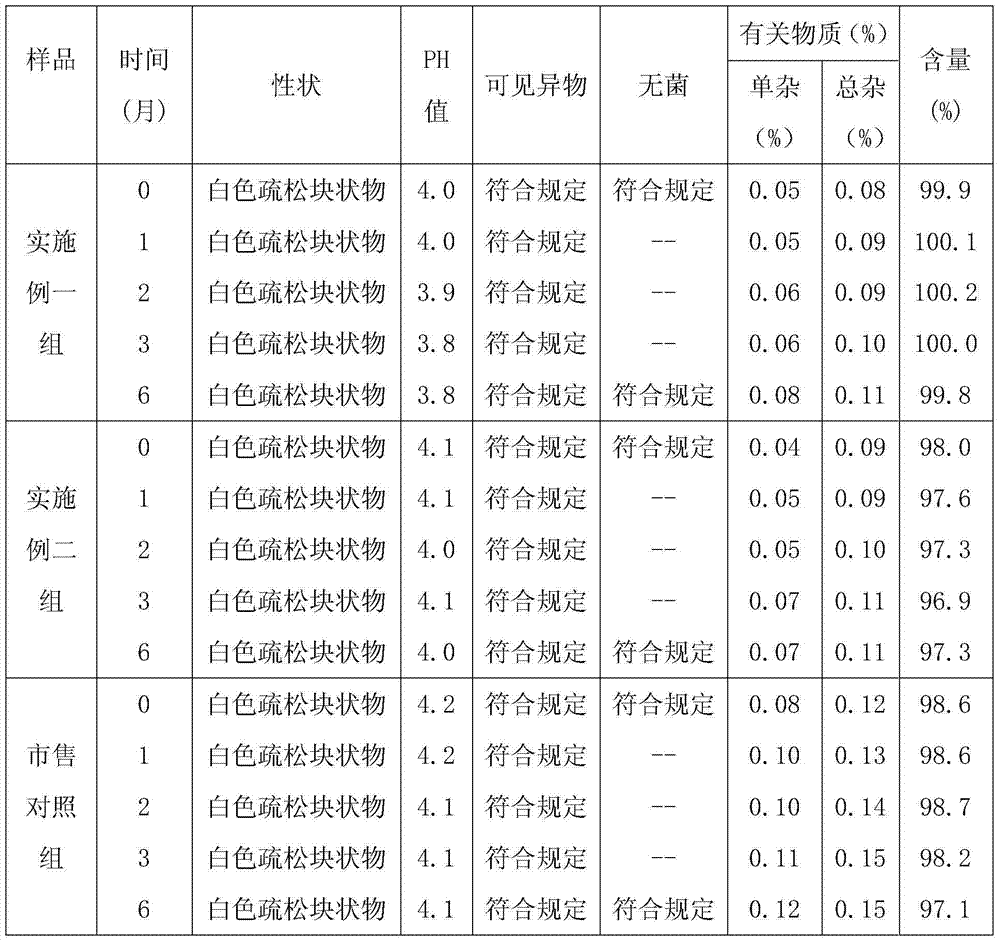
Bromhexine hydrochloride composition freeze-dried powder for injection
A technology of bromhexine hydrochloride and freeze-dried powder injection, which is applied in the field of medicine and medicine manufacturing, can solve the problems of high risk of clinical use and acute renal failure, and achieve the goal of avoiding renal failure, enhancing solubility and high stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Preparation of bromhexine hydrochloride composition freeze-dried powder for injection (specification: 4 mg, calculated as bromhexine hydrochloride), calculated in 1000 vials.
[0041] 1. Prescription:
[0042] Bromhexine Hydrochloride 4g
[0043] Chitosan Nanoparticles 3.6g
[0044] Water for injection 2000ml
[0045] 2. Preparation process:
[0046] (1) Slowly add 3.6g of chitosan nanoparticles into the prescribed amount of water for injection (theoretical loading is 2ml / bottle), and stir until dissolved while adding.
[0047] (2) Continue to add 4g of bromhexine hydrochloride and stir to dissolve until clear.
[0048] (3) Use sodium hydroxide to adjust the pH to 4.0, add activated carbon with 0.1% volume of water for injection and stir for 30 minutes; then conduct decarburization circulation and filtration through titanium rods for 30 minutes; then conduct sterilization cycles through 0.45 μm and 0.22 μm Filtrate for 30 minutes; detect the content of t...
Embodiment 2
[0050] Example 2: Preparation of bromhexine hydrochloride composition freeze-dried powder for injection (specification: 4 mg, calculated as bromhexine hydrochloride), calculated in 1000 vials.
[0051] 1. Prescription:
[0052] Bromhexine Hydrochloride 4g
[0053] Chitosan Nanoparticles 4g
[0054] Water for injection 2000ml
[0055] 2. Preparation process:
[0056] (1) Slowly add 4g of chitosan nanoparticles into the prescribed amount of water for injection (theoretical loading is 2ml / bottle), and stir until dissolved while adding.
[0057] (2) Continue to add 4g of bromhexine hydrochloride and stir to dissolve until clear.
[0058] (3) Use sodium hydroxide to adjust the pH to 4.0, add activated carbon with 0.1% volume of water for injection and stir for 30 minutes; then conduct decarburization circulation and filtration through titanium rods for 30 minutes; then conduct sterilization cycles through 0.45 μm and 0.22 μm Filtrate for 30 minutes; detect the content of the int...
Embodiment 3
[0060] Example 3. Preparation of bromhexine hydrochloride composition freeze-dried powder for injection (specification: 4 mg, calculated as bromhexine hydrochloride), calculated in 1000 vials.
[0061] 1. Prescription:
[0062] Bromhexine Hydrochloride 4g
[0063] Chitosan Nanoparticles 3.2g
[0064] Water for injection 2000ml
[0065] 2. Preparation process:
[0066] (1) Slowly add 3.2g of chitosan nanoparticles into the prescribed amount of water for injection (theoretical loading is 2ml / bottle), and stir until dissolved while adding.
[0067] (2) Continue to add 4g of bromhexine hydrochloride and stir to dissolve until clear.
[0068] (3) Use sodium hydroxide to adjust the pH to 4.0, add activated carbon with 0.1% volume of water for injection and stir for 30 minutes; then conduct decarburization circulation and filtration through titanium rods for 30 minutes; then conduct sterilization cycles through 0.45 μm and 0.22 μm Filtrate for 30 minutes; detect the content of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com