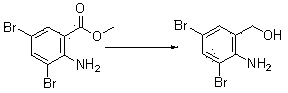Production method of bromhexine hydrochloride
A technology of bromhexine hydrochloride and a production method, applied in the production field of bromhexine hydrochloride, can solve the problems of difficult to scale up production and high equipment requirements, and achieve the effects of less generation of by-products, low raw material requirements, and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Synthesis of 3,5-dibromo-2-aminobenzyl alcohol (II)
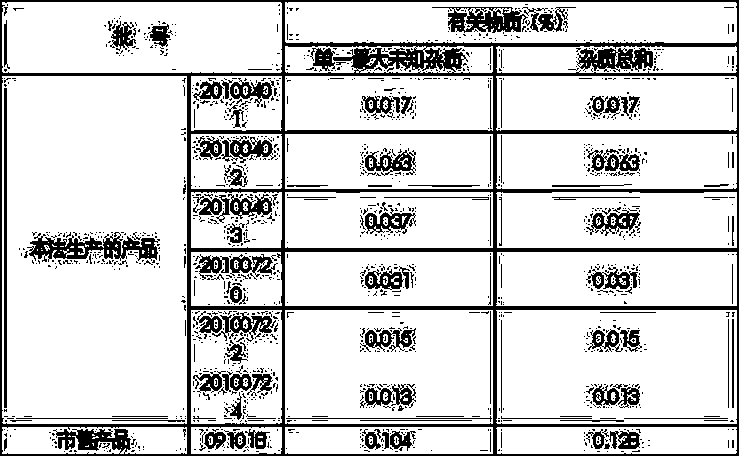
[0030] Add 220Kg of polyethylene glycol 400 to the reaction kettle, add 25Kg of 3,5-dibromo-2-aminobenzoic acid methyl ester, stir and mix well, add 9Kg of sodium borohydride, heat up to 70°C, and stir for 5 hours . After cooling to room temperature, the reaction solution was diluted with 5 times the volume of water, and an off-white solid was precipitated. Shake the filter, and wash the filter cake with water until neutral. Dry off-white solid 3,5-dibromo-2-aminobenzyl alcohol (II): 20Kg, yield 88%.
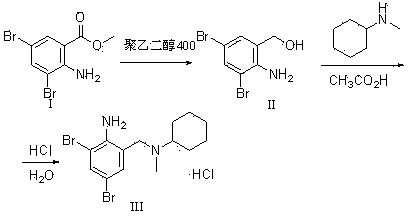
[0031] 2. Synthesis of bromhexine hydrochloride (III)
[0032] Add 25Kg of N-methylcyclohexylamine into the reaction kettle, add 20Kg of 3,5-dibromo-2-aminobenzyl alcohol under stirring, then add 11Kg of acetic acid, and heat up to reflux for 10 hours. Cool to room temperature, dilute with water while stirring, then add hydrochloric acid and ethyl acetate. Stir, spin filter, wash the filter cake with water, the...
Embodiment 2
[0036] 1. Synthesis of 3,5-dibromo-2-aminobenzyl alcohol (II)
[0037] Add 220Kg of polyethylene glycol 400 to the reaction kettle, add 25Kg of 3,5-dibromo-2-aminobenzoic acid methyl ester, stir and mix well, add 9Kg of sodium borohydride, heat up to 70°C, and stir for 5 hours . After cooling to room temperature, the reaction solution was diluted with water, and an off-white solid was precipitated. Shake the filter, and wash the filter cake with water until neutral. Dry off-white solid 3,5-dibromo-2-aminobenzyl alcohol (II): 20.2Kg, yield 88.9%.
[0038] 2. Synthesis of bromhexine hydrochloride (III)
[0039] Add 25Kg of N-methylcyclohexylamine into the reaction kettle, add 20.2Kg of 3,5-dibromo-2-aminobenzyl alcohol under stirring, then add 11Kg of acetic acid, and heat up to reflux for 10 hours. Cool to room temperature, dilute with water while stirring, then add hydrochloric acid and ethyl acetate. Stir, shake and filter, wash the filter cake with water, wash with acet...
Embodiment 3
[0043] 1. Synthesis of 3,5-dibromo-2-aminobenzyl alcohol (II)
[0044] Add 220Kg of polyethylene glycol 400 into the reaction kettle, add 25Kg of 3,5-dibromo-2-aminobenzoic acid methyl ester, stir and mix, add potassium borohydride 9.5Kg, heat up to 70°C, and stir for 5 Hour. After cooling to room temperature, the reaction solution was diluted with water, and an off-white solid was precipitated. Shake the filter, and wash the filter cake with water until neutral. Dry off-white solid 3,5-dibromo-2-aminobenzyl alcohol (II): 19.7Kg, yield 86.7%.
[0045] 2. Synthesis of bromhexine hydrochloride (III)
[0046] Add 25Kg of N-methylcyclohexylamine into the reaction kettle, add 19.7Kg of 3,5-dibromo-2-aminobenzyl alcohol under stirring, then add 11Kg of acetic acid, and heat up to reflux for 10 hours. Cool to room temperature, dilute with water while stirring, then add hydrochloric acid and ethyl acetate. Stir, spin filter, wash the filter cake with water, then with ethyl acetat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com