Tinidazole and sodium chloride injection and preparation method thereof
A technology of nidazole sodium chloride and injection, which is applied in the fields of pharmaceutical formula, drug delivery, inorganic non-active ingredients, etc. It can solve the problem of easy crystallization, low pass rate of clarity of tinidazole sodium chloride injection, and influence on the yield of finished products. Problems such as production rate and sales market, to achieve the effect of solving crystallization problems, improving drug stability and clinical drug safety, and solving instability problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
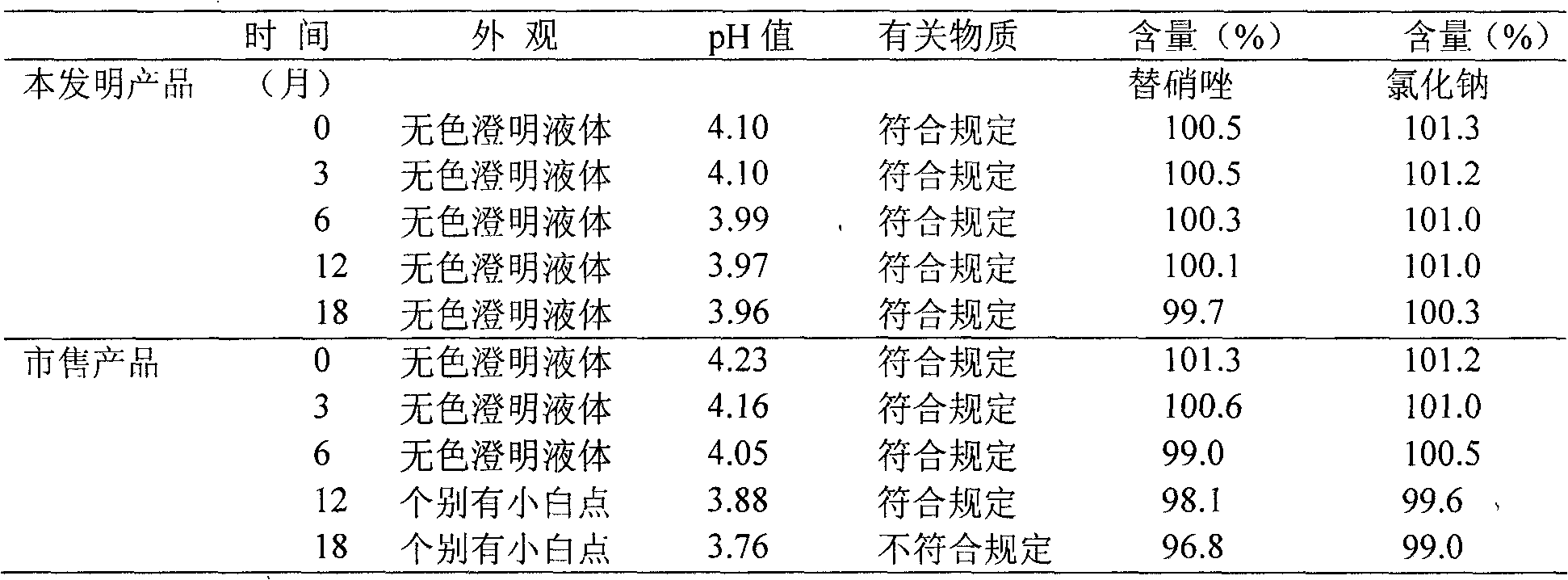
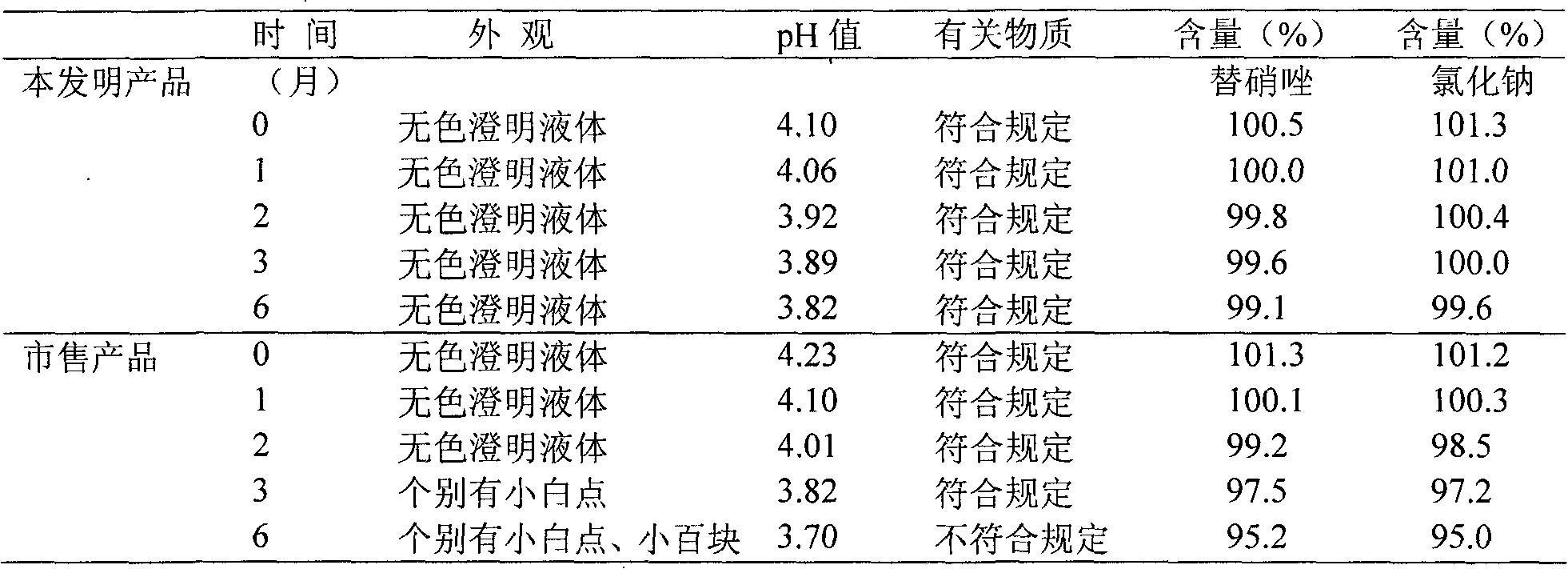
Image
Examples
Embodiment 1
[0031]
[0032] Weigh each raw material according to the formula for subsequent use. The sodium chloride of formula quantity is placed in the concentrated preparation tank that is placed in appropriate amount of water for injection, stirs and dissolves, makes sodium chloride become the 20% concentrated solution measured by weight / volume. For the first time, add 0.1 g of activated carbon for needles, stir and mix well, boil for 15 minutes, and let cool to 80°C. Use a titanium rod filter to filter under pressure, circulate it for 10 minutes, and transfer it to a dilute mixing pot.
[0033] Put the prescribed amount of tinidazole into a clean container, add 40ml of water for injection, stir for 15 minutes to fully dissolve it, and then transfer it to a dilute mixing pot. Then put 0.1 g of edetate calcium sodium into the dilute mixing pot, and add water for injection to 80% of the total amount in the dilute mixing pot. Add 0.1 g of activated carbon for needles for the second ...
Embodiment 2
[0043]
[0044] Weigh each raw material according to the formula for subsequent use. The sodium chloride of formula quantity is placed in the concentrated preparation tank that is placed in appropriate amount of water for injection, stirs and dissolves, makes sodium chloride become the 20% concentrated solution measured by weight / volume. For the first time, add 0.1 g of activated carbon for needles, stir and mix well, boil for 15 minutes, and let cool to 80°C. Use a titanium rod filter to filter under pressure, circulate it for 10 minutes, and pump it into the dilute pot.
[0045] Pour the prescribed amount of tinidazole into a clean container, add 40ml of water for injection, stir for 15 minutes to fully dissolve, and then add it to the dilute mixing pot. Then 0.2 g of trisodium citrate is dropped into the dilute mixing pot, and water for injection is added to the full amount of 80% in the dilute mixing pot. Add 0.1 g of activated carbon for needles for the second time, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com