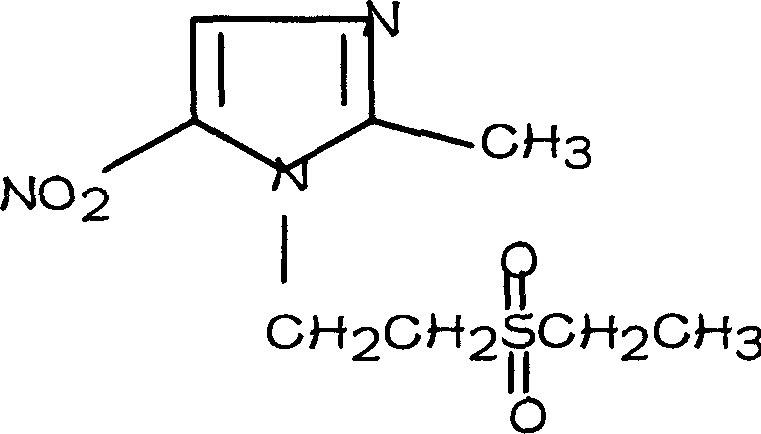
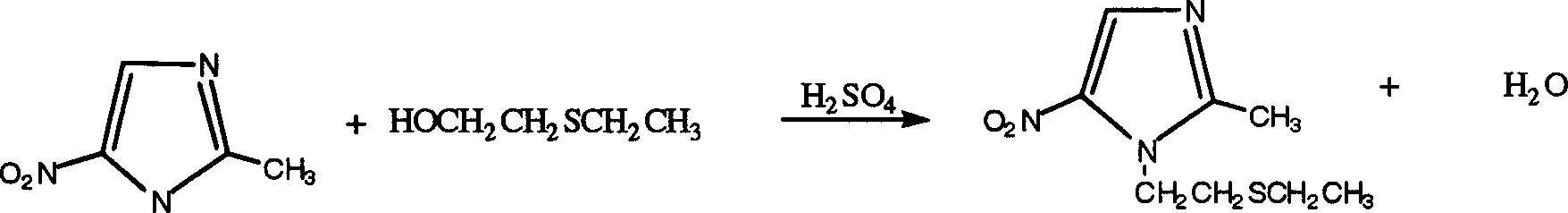Tinidazole preparing process
A technology for tinidazole and nitroimidazole, which is applied in the field of chemical pharmacy, can solve the problems of numerous raw materials, long production cycle, low yield and the like, and achieve the effects of shortening reaction steps, reducing mass usage and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] 2, the preparation of tinidazole
[0025]
[0026] General Formula IV General Formula I
Embodiment 1
[0028] 1. Add 170g (1.35mol) of 2-methyl-5-nitroimidazole and 106g (1.0mol) of β-hydroxy ethyl sulfide into a 500ml three-necked flask, then add 200ml of xylene, heat up, and heat up at 30 Slowly add 75ml (1.38mol) of concentrated sulfuric acid dropwise at ~55°C for 3-5 hours, then raise the temperature to about 70°C, and keep the reaction at about 70°C for 6 hours. After the heat preservation is completed, the temperature is lowered to below 50°C, the pH of the reaction solution is adjusted to 6.5-7.5 with 20-22% ammonia water, the solid is removed by filtration, the layers are separated, and the organic layer is taken.
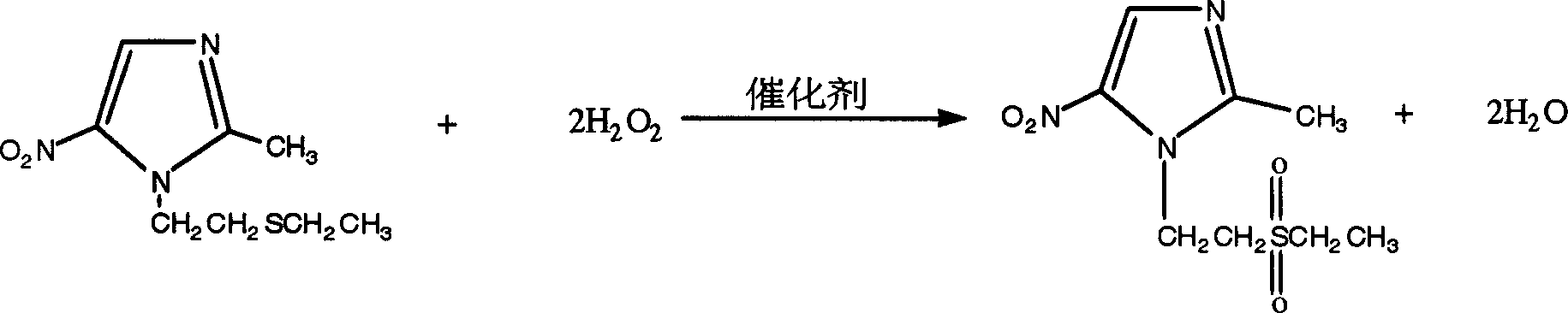
[0029] 2. Add 50ml of water and a catalytic amount of ammonium molybdate to the organic layer, raise the temperature to about 65°C and slowly add about 250g of hydrogen peroxide dropwise, keep warm at about 65°C for 3 hours, cool to below 35°C, and filter to obtain about 170g of hydrogen peroxide Tinidazole crude product, the crude product was refined and dr...
Embodiment 2
[0031] 1. Add 170g (1.35mol) of 2-methyl-5-nitroimidazole and 106g (1.0mol) of β-hydroxyethyl sulfide into a 500ml three-necked flask, then add 200ml of o-xylene, heat up, and Slowly add 122ml (2.24mol) of concentrated sulfuric acid dropwise at 40-60°C for 3-5 hours, then raise the temperature to about 70°C, and keep the reaction at about 70°C for 6 hours. After the heat preservation is completed, the temperature is lowered to below 50°C, the pH of the reaction solution is adjusted to 6.5-7.5 with 20-22% ammonia water, the solid is removed by filtration, the layers are separated, and the organic layer is taken.
[0032] 2. Add 50ml of water and a catalytic amount of ammonium molybdate to the organic layer, raise the temperature to about 60°C and slowly add about 250g of hydrogen peroxide dropwise, keep warm at about 60°C for 3 hours, cool to below 35°C, and filter to obtain about 170g of hydrogen peroxide Tinidazole crude product, the crude product was refined and dried with 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com