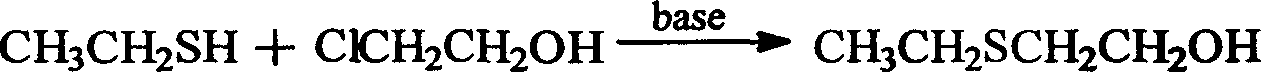Preparation methoh of tinidazole
A technology of tinidazole and nitroimidazole, which is applied in the field of preparation of antifungal drug tinidazole, can solve the problems of low yield, black color of intermediate products, high price, etc., and achieve increased solvent recovery and reduced dosage , The effect of production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] 62g (1.0mol) ethanethiol is added in the 500ml reaction bottle, slowly add the industrial sodium methylate of 200g (1.04mol) 28%, the solution becomes pale yellow immediately, keep at room temperature then, slowly add dropwise by 80.6g (1.0 mol) of chloroethanol and 50ml methanol, the dropwise addition was completed, reacted at room temperature for 1 hour, then slowly heated to 80 ° C, and reacted for 3 hours. The cake was rinsed with methanol, dried for later use, the mother liquor was distilled to recover the methanol, and the residue was distilled under reduced pressure to collect fractions at 101-103°C / 50mmHg. 96.5%.
Embodiment 2
[0014] The sodium methoxide was replaced by an ethanol solution of sodium ethoxide, and the ethanol solution of chloroethanol was added dropwise. Other operations were the same as in Example 1 to obtain 101.8 g of β-hydroxyethyl sulfide, with a yield of 96%.
Embodiment 3
[0016] The sodium methoxide was replaced with a tert-butanol solution of sodium tert-butoxide, and pure chloroethanol was added dropwise. The other operations were the same as in Example 1 to obtain 103.1 g of β-hydroxyethyl sulfide with a yield of 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com