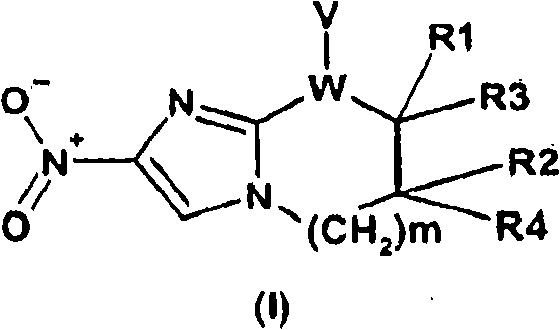
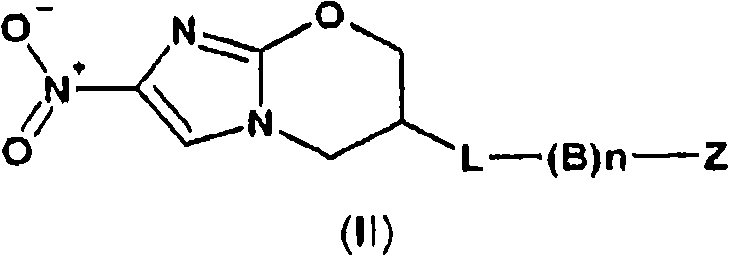
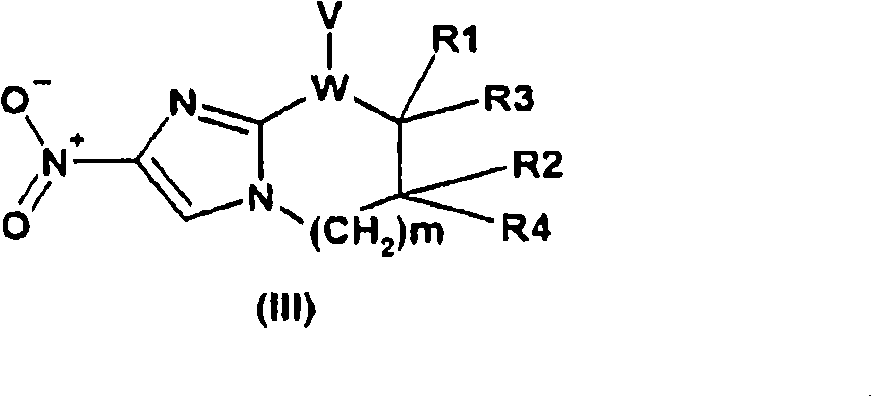
Nitroimidazole compounds
A technology of compounds and groups, applied in the fields of organic chemistry, antibacterial drugs, organic active ingredients, etc., can solve the problems of complex tablet formulations, expensive synthetic routes, low solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0303] (S)-1-(tert-butyldimethylsilyloxy)-3-(2-chloro-4-nitro-imidazol-1-yl)-propan-2-ol (3)
[0304]
[0305] Dissolve 2-chloro-4-nitro-imidazole (20.0 g, 0.14 mol, 100 mol%) in anhydrous EtOH (200 mL), add anhydrous K 2 CO 3 (2.82g, 0.020mol, 15mol%), followed by tert-butyl-dimethyl-((S)-1-oxiranylmethoxy)-silane (22.2mL, 0.11mol, 0.78mol%) . The reaction mixture was heated to 70 °C for 6-10 h. The solvent was then removed in vacuo and the reaction mixture was dissolved in ethyl acetate. The organic layer was washed several times with water, 0.5 N HCI, water, brine, and the solvent was removed in vacuo to give crude alcohol as a yellowish solid. The solid was suspended in ether and filtered to give the final product as a colorless powder. The remaining filtrate was concentrated and the process of settling the product with diethyl ether was repeated twice.
[0306] MS: M + 336.3.
[0307] Melting point: 116-118°C.
[0308] [α] 21 D =-29.43 (C=0.003, MeOH).
Embodiment 2
[0310] 1-[(S)-3-(tert-butyl-dimethyl-silyloxy)-2-(tetrahydro-pyran-2-yloxy)-propyl]-2- Chloro-4-nitro-1H-imidazole (4)
[0311]
[0312] (S)-1-(tert-butyl-dimethyl-silyloxy)-3-(2-chloro-4-nitro-imidazol-1-yl)-propan-2-ol (3.0g, 8.9mmol, 100mol%) was dissolved in dichloromethane (100mL), and freshly distilled 3,4-dihydro-2H-pyran (1.5g, 17.8mmol, 200mol%) was added to the solution, followed by p-toluene Pyridinium sulfonate (3.4 g, 13.4 mmol, 150 mol%). The reaction mixture was stirred at room temperature for 24 h. The reaction mixture was quenched with saturated aqueous sodium bicarbonate. The organic layer was separated and the aqueous portion was extracted with dichloromethane. The combined organic layers were washed with water, brine, and washed with MgSO 4 After drying, the solvent was removed in vacuo to give 1-[(S)-3-(tert-butyl-dimethyl-silanyloxy)-2-(tetrahydro-pyran-2-yl as a colorless oil Oxy)-propyl]-2-chloro-4-nitro-1H-imidazole.
[0313] MS: M + 420....
Embodiment 3
[0315] (S)-2-nitro-6-(tetrahydro-pyran-2-yloxy)-6,7-dihydro-5H-imidazo[2,1-b]-[1,3]- evil Zinc (5)
[0316]
[0317] 1-[(S)-3-(tert-butyl-dimethyl-silyloxy)-2-(tetrahydro-pyran-2-yloxy)-propyl]-2-chloro-4 -Nitro-1H-imidazole (0.74g, 1.76mmol, 100mol%) was dissolved in anhydrous THF (180mL), and TBAF (1M THF solution, 1.76mL, 100mol%) was added to the above solution. The reaction tube was sealed and exposed to microwaves at 140 °C for 7 min. The solvent was removed under vacuum and the residue was purified on silica gel to give (S)-2-nitro-6-(tetrahydro-pyran-2-yloxy)-6,7-dihydro as a yellowish oil -5H-imidazo[2,1-b]-[1,3]oxazine.
[0318] (S)-3-(2-Chloro-4-nitro-imidazol-1-yl)-2-(tetrahydro-pyran-2-yloxy)-propan-1-ol (0.053g, 0.172 mmol, 100 mol%) was dissolved in anhydrous tetrahydrofuran (17 mL), and TBAF (1M THF solution, 0.17 mL, 100 mol%) was added to the solution. The reaction tube was sealed and exposed to microwaves at 140 °C for 7 min. The solvent was rem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com