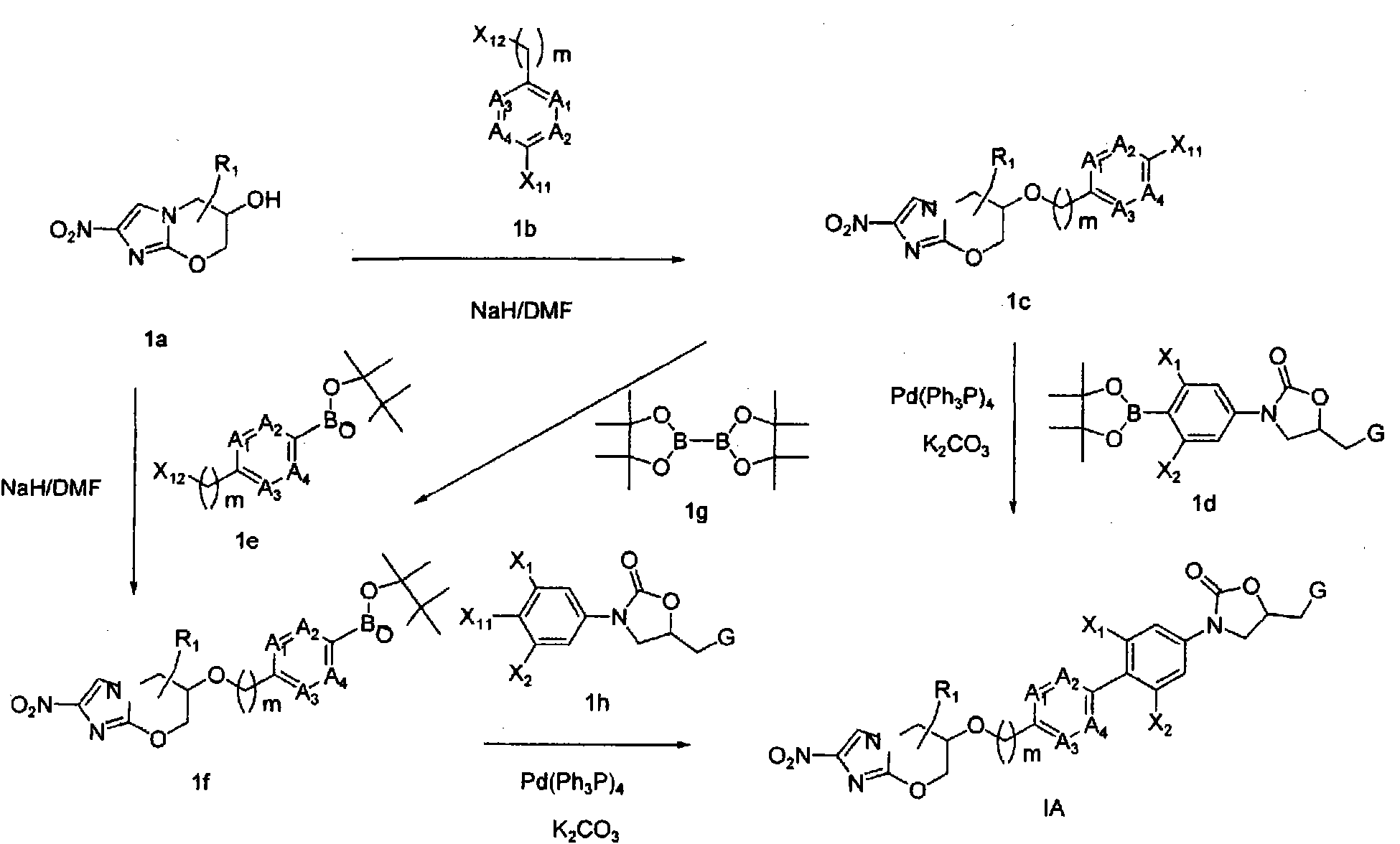
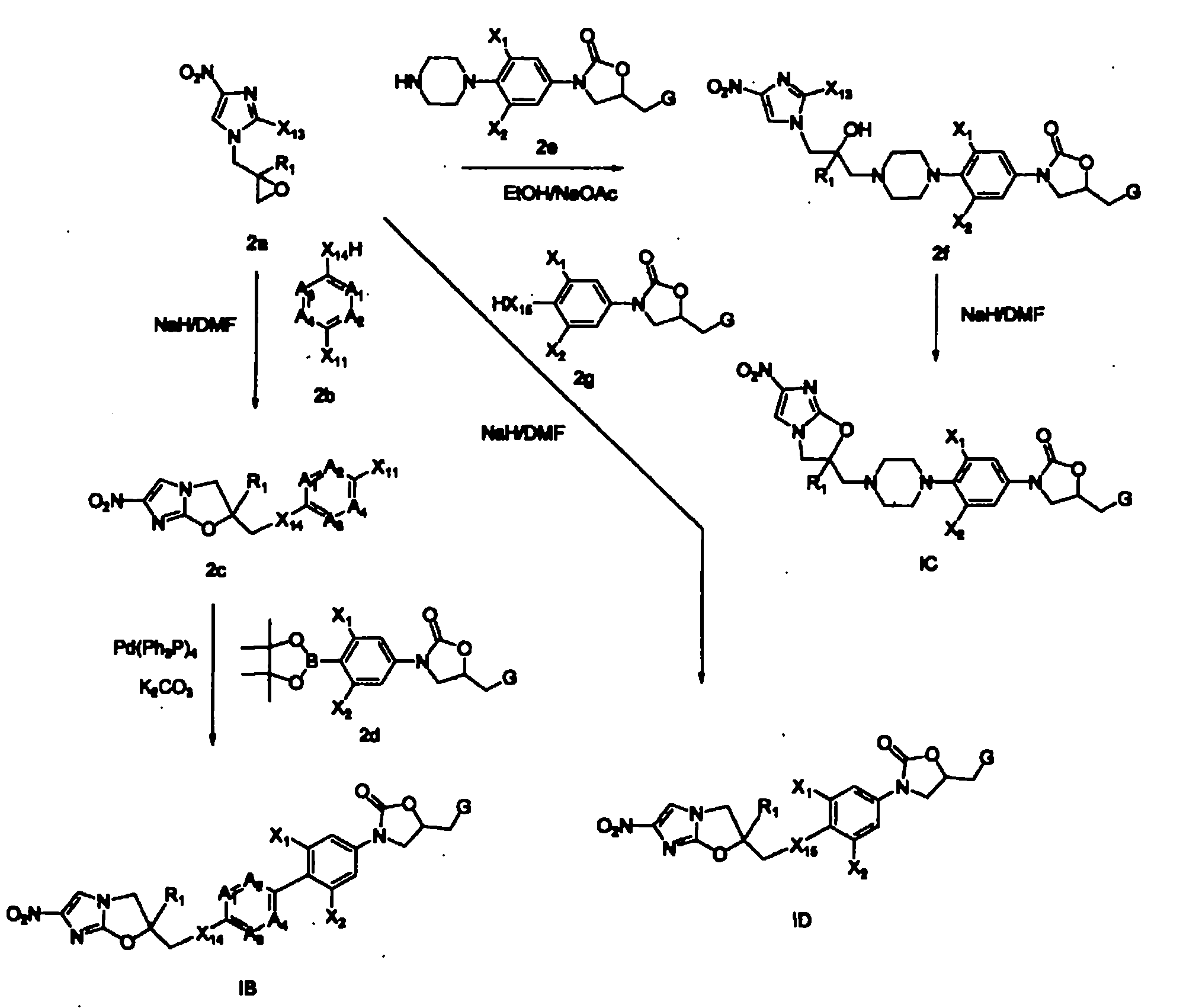
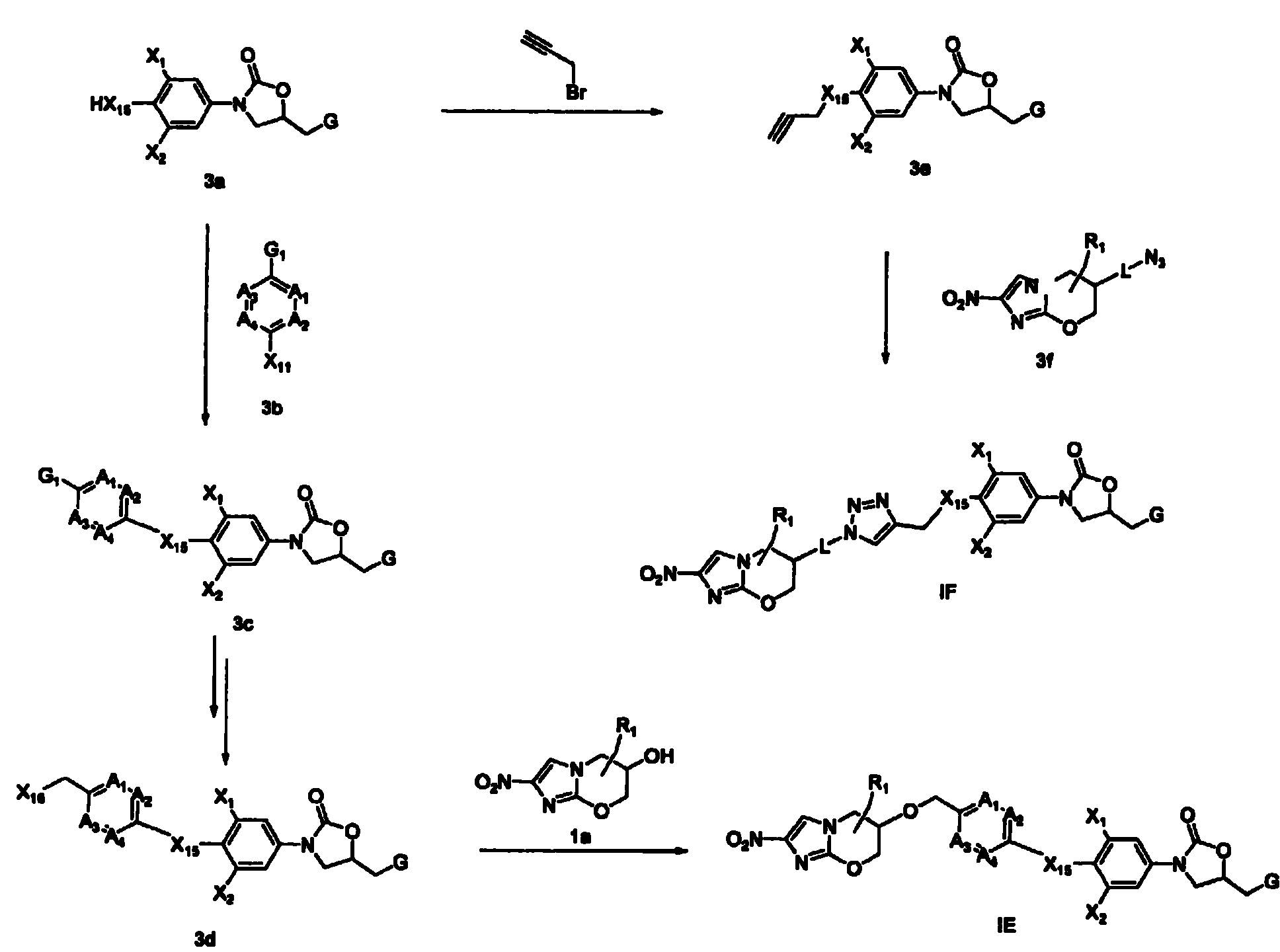
Bicyclic nitroimidazoles covalently linked to substituted phenyl oxazolidinones
A nitroimidazole ring, unsubstituted technology, applied in the field of bicyclic nitroimidazoles covalently linked to substituted phenyl oxazolidinones, which can solve the problems of no references to be published
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] (S, S)-N-{3-[3-fluoro-4-(4-{2-[4-(2-nitro-6,7-dihydro-5H-imidazo[2,1-b ][1,3] Oxyzin-6-yloxymethyl)-phenoxy]-acetyl}-piperazin-1-yl)-phenyl]-2-oxo- Oxazolidin-5-ylmethyl}-acetamide:
[0133]
[0134] Step 1. (4-Hydroxymethyl-phenoxy)-tert-butyl acetate. 4-Hydroxymethylphenol (3.70g, 30mmol), tert-butyl bromoacetate (6.0mL, 40mmol), K 2 CO 3 (16.6g, 120mmol) suspension in acetonitrile (100mL) in N 2 Stirred at 60°C for 16h. The reaction mixture was filtered and the solvent was removed under reduced pressure to give a crude product which was purified by flash chromatography with EtOAc / hexanes to give the title product as an oil (6.22 g, 87%).
[0135] 1 H NMR (400MHz, CDCl 3 )δ7.29(d, J=8.0Hz, 2H), 6.88(d, J=8.4Hz, 2H), 4.62(d, J=5.6Hz, 2H), 4.52(s, 2H), 1.49(s, 9H).
[0136] Step 2. (4-Chloromethyl-phenoxy)-tert-butyl acetate. at 0°C in N 2 To a stirred solution of (4-hydroxymethyl-phenoxy)-tert-butyl acetate (2.38 g, 10 mmol) in dichloromethane (50 mL) ...
Embodiment 2
[0142] (S,S)-N-(3-{3-fluoro-4-[4-(2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3] Oxyzin-6-yloxymethyl)-benzyloxy]-phenyl}-2-oxo- Oxazolidin-5-ylmethyl)-acetamide:
[0143]
[0144] Step 1.6 (S)-(4-Chloromethyl-benzyloxy)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3] Zinc. To stirred 2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3] To a solution of azin-6(S)-ol (0.1 g, 0.54 mmol) in anhydrous DMF (1 mL) was added NaH (60% dispersion in oil, 26.0 mg, 0.65 mmol) and stirred at 0 °C 30 minutes. 1,4-Dichloromethylbenzene (472 mg, 2.7 mmol) in anhydrous DMF (0.5 mL) was added to the reaction mixture and stirred at 0 °C for 30 minutes and at room temperature for another 3 hours. The reaction mixture was diluted with ethyl acetate, washed with water and saturated brine. Anhydrous Na for organic layer 2 SO 4 Dry, filter and evaporate. The residue was washed with hexane to obtain the title compound (65 mg, 37%) as a solid.
[0145] ESI MS m / z 324 (M+H + ); 1 H NMR (400MHz, CDCl 3 ...
Embodiment 3
[0149] (S,S)-N-(3-{3-fluoro-4-[3-(2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3] Oxyzin-6-yloxymethyl)-benzyloxy]-phenyl}-2-oxo- Oxazolidin-5-ylmethyl)-acetamide:
[0150]
[0151] Step 1.6 (S)-(3-Chloromethyl-benzyloxy)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3] Zinc. The title compound was prepared by the same method as described in the preparation of Example 2, except that 1,3-bis-(chloromethyl)-benzene was used in place of 1,4-bis-(chloromethyl)-benzene.
[0152] ESI MS m / z 473 (M+H + ); 1 HNMR (400MHz, CDCl 3 )δ7.38(s, 1H), 7.32-7.19(m, 4H), 4.71(d, J=30Hz, 1H), 4.60(d, J=21Hz, 2H), 4.33(d, J=30.0Hz, 1H), 4.15(m, 3H), 3.45(s, 2H), 3.41(s, 4H), 2.37(s, 4H), 1.44(s, 9H).
[0153] Step 2. (S,S)-N-(3-{3-fluoro-4-[3-(2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1 ,3] Oxyzin-6-yloxymethyl)-benzyloxy]-phenyl}-2-oxo- oxazolidin-5-ylmethyl)-acetamide. The title compound was prepared by the same method as described in the preparation of Example 2, except that the starting m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com