Ornidazole optical antimer preparation and purification method
An ornidazole optical and enantiomer technology is applied in the field of preparation and purification of ornidazole optical enantiomer, can solve the problems of inapplicability to large-scale production, high cost and the like, and achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
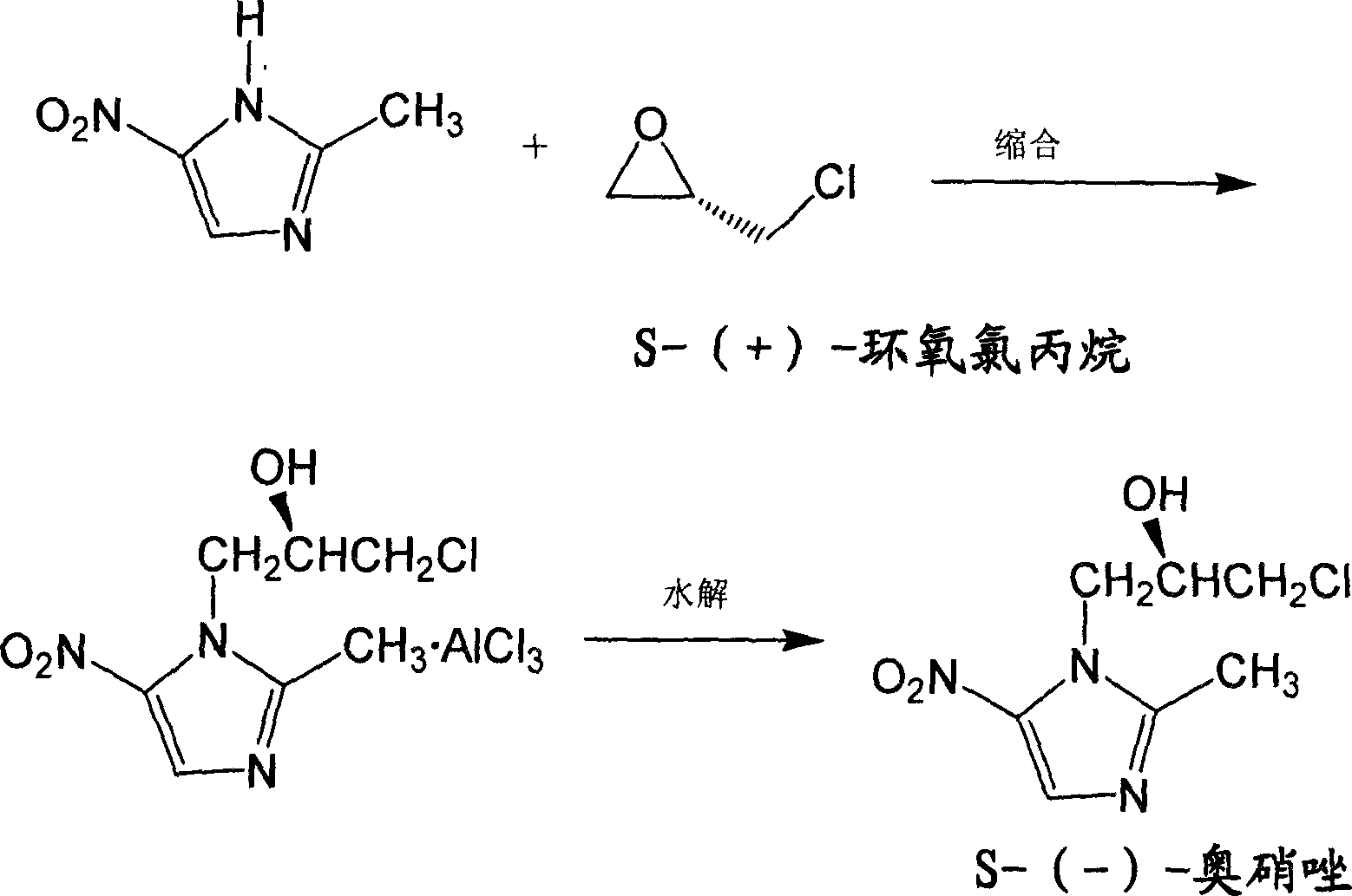
[0020] Put 500 liters of ethyl acetate and 52.5 kg of 2-methyl-5-nitroimidazole into a dry 1000-liter enamel reaction pot, stir and drop to 0°C, add 80 kg of aluminum chloride into the reaction pot in batches, Control the temperature not to exceed 10°C, cool down to 5°C after the addition, and keep stirring for 1 hour; add 50 liters of S-(+)-epichlorohydrin dropwise into the reaction pot, control the temperature not to exceed 10°C, 5-10 ℃ heat preservation reaction for 2.5 hours; slowly add 300 liters of ice water to the reaction solution, control the temperature not to exceed 30 ℃, after the addition is completed, 20-30 ℃ heat preservation reaction for 1 hour; filter the reaction liquid, and the filtrate is allowed to stand and separate Take the organic phase and add 200 liters of water, then stir and add about 50 liters of concentrated hydrochloric acid to make the pH value 1.0, let it stand for stratification, take the water phase and add 500 liters of ethyl acetate, add amm...
Embodiment 2
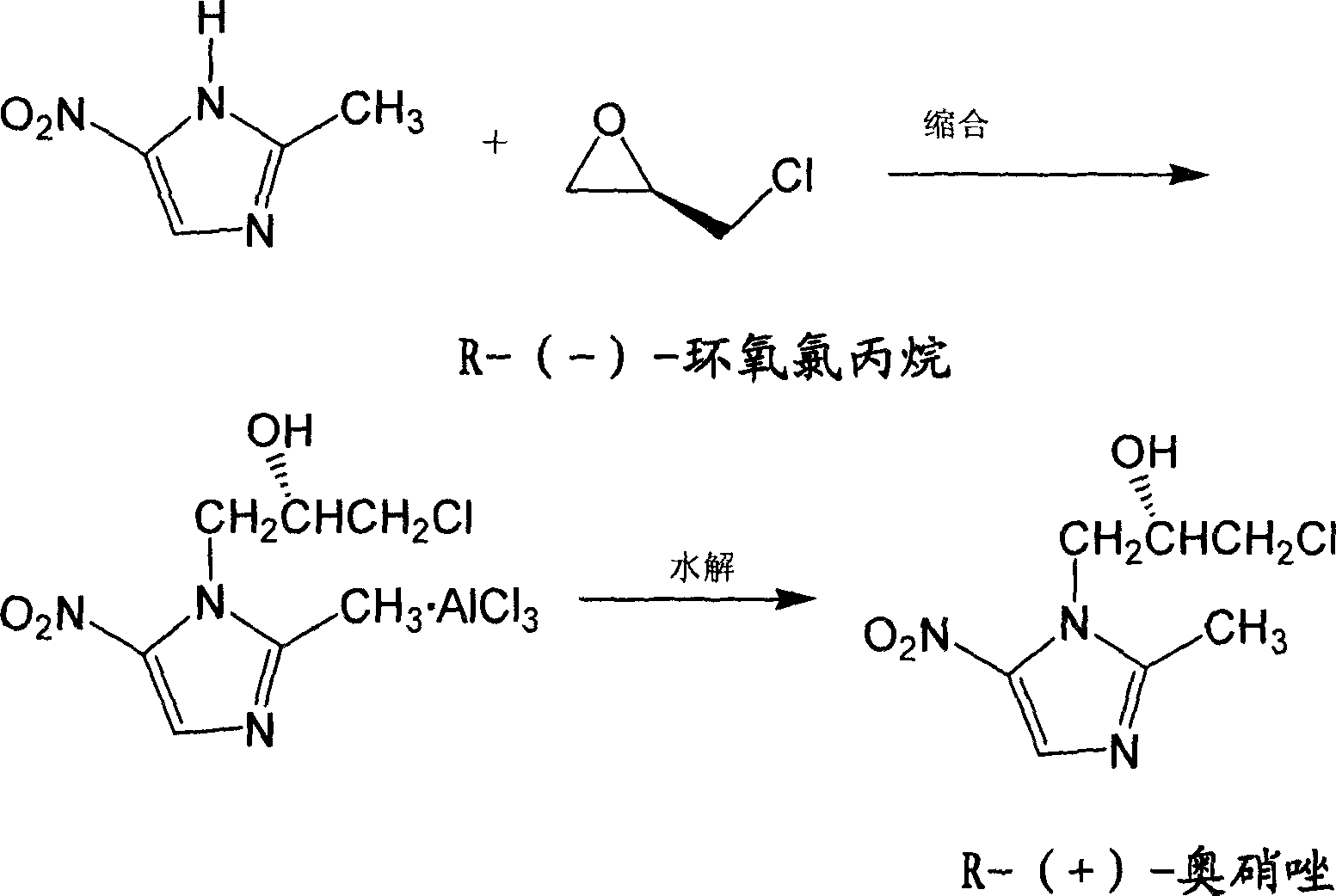
[0022] Put 500 liters of ethyl acetate and 52.5 kilograms (kg) of 2-methyl-5-nitroimidazole into a dry 1000-liter enamel reaction pot, stir it down to 0 °C, and add 80 kg of ferric chloride to the reaction pot in batches Inside, control the temperature not to exceed 10°C, cool down to 5°C after the addition, and keep stirring for 1 hour; add 50 liters of R-(-)-epichlorohydrin dropwise into the reaction pot, control the temperature not to exceed 10°C, add After the completion of the reaction at 5-10 ° C for 3 hours; slowly add 300 liters of ice water to the reaction solution, control the temperature not to exceed 30 ° C, after the addition is completed, keep the reaction at 20-30 ° C for 1.5 hours; filter the reaction solution and let the filtrate stand Layering, take the organic phase and add 200 liters of water, then stir and add about 50 liters of concentrated hydrochloric acid to make the pH value 1.5, let it stand for stratification, take the water phase, add 500 liters of ...
Embodiment 3
[0024] Purification of products. Put 200 grams (g) of S-(-)-ornidazole crude product (impurity 13%) and 2000 milliliters of toluene into the reaction flask as prepared in Example 1, stir and heat up to 60 ° C, keep stirring for 15 minutes and then filter while hot , the filtrate was placed at -5°C for 12 hours to crystallize, filtered to obtain 160g of solid (impurity 2%), put the dried solid and 128 ml of 75% ethanol into the reaction flask, stirred and raised the temperature to 55°C, and added activated carbon after the solid was dissolved 2 g, kept stirring for 20 minutes and then filtered while hot. The filtrate was placed at 5°C for 12 hours to crystallize, filtered, and the resulting solid was rinsed with cold ethanol and dried to obtain 112 g of S-(-)-ornidazole (0.2% impurity).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com