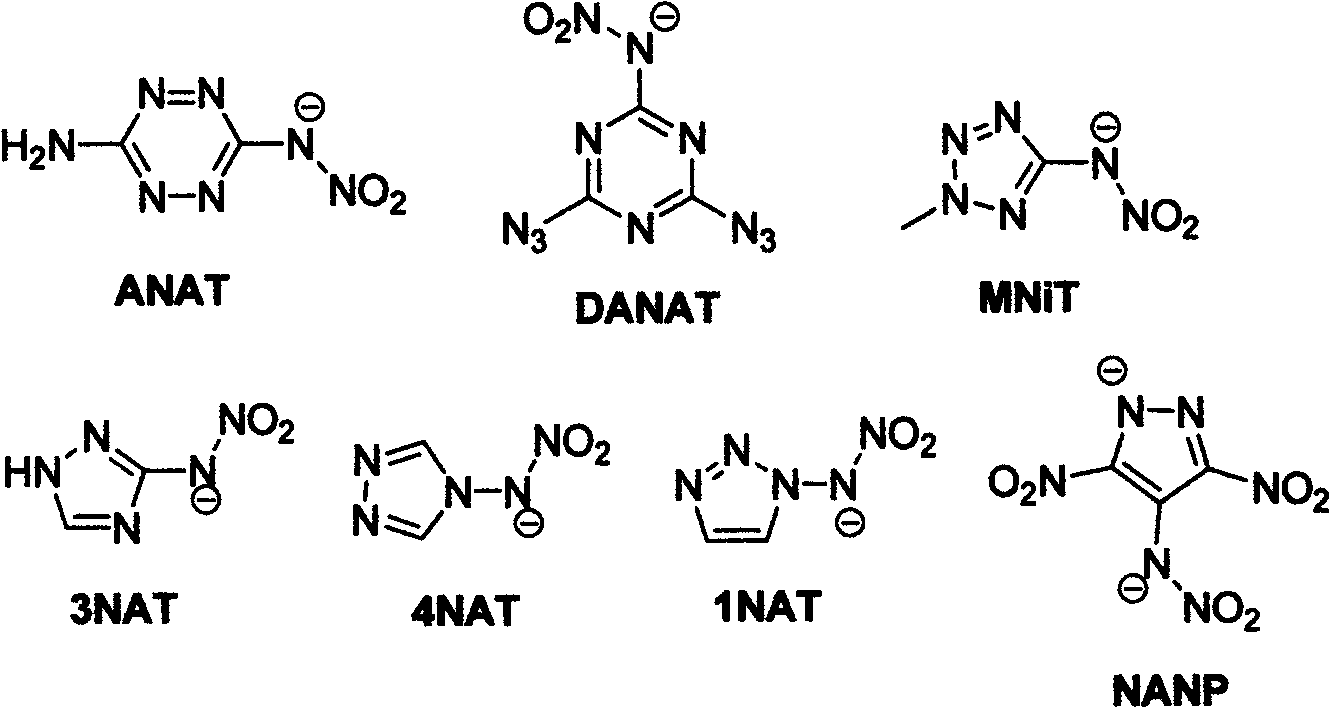
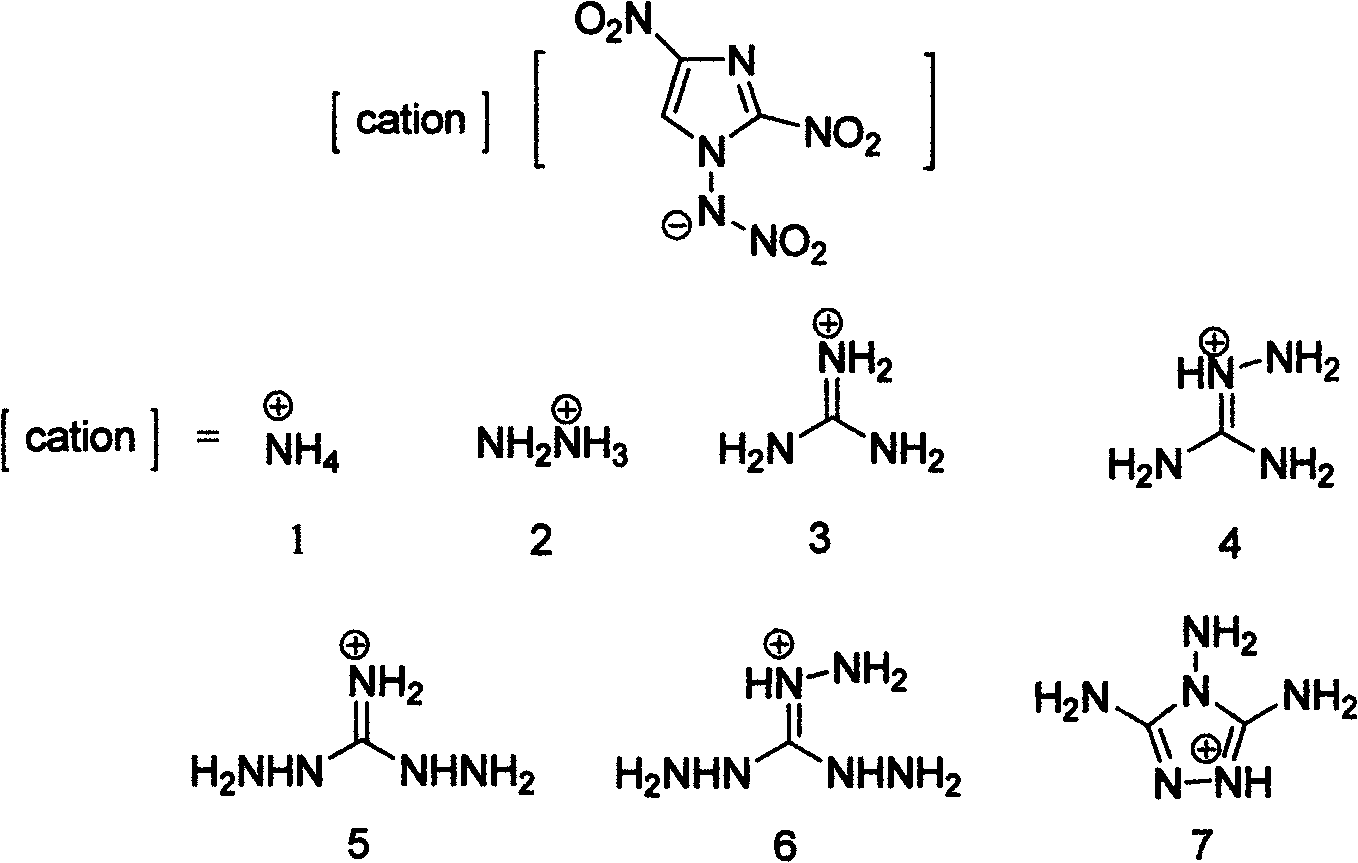
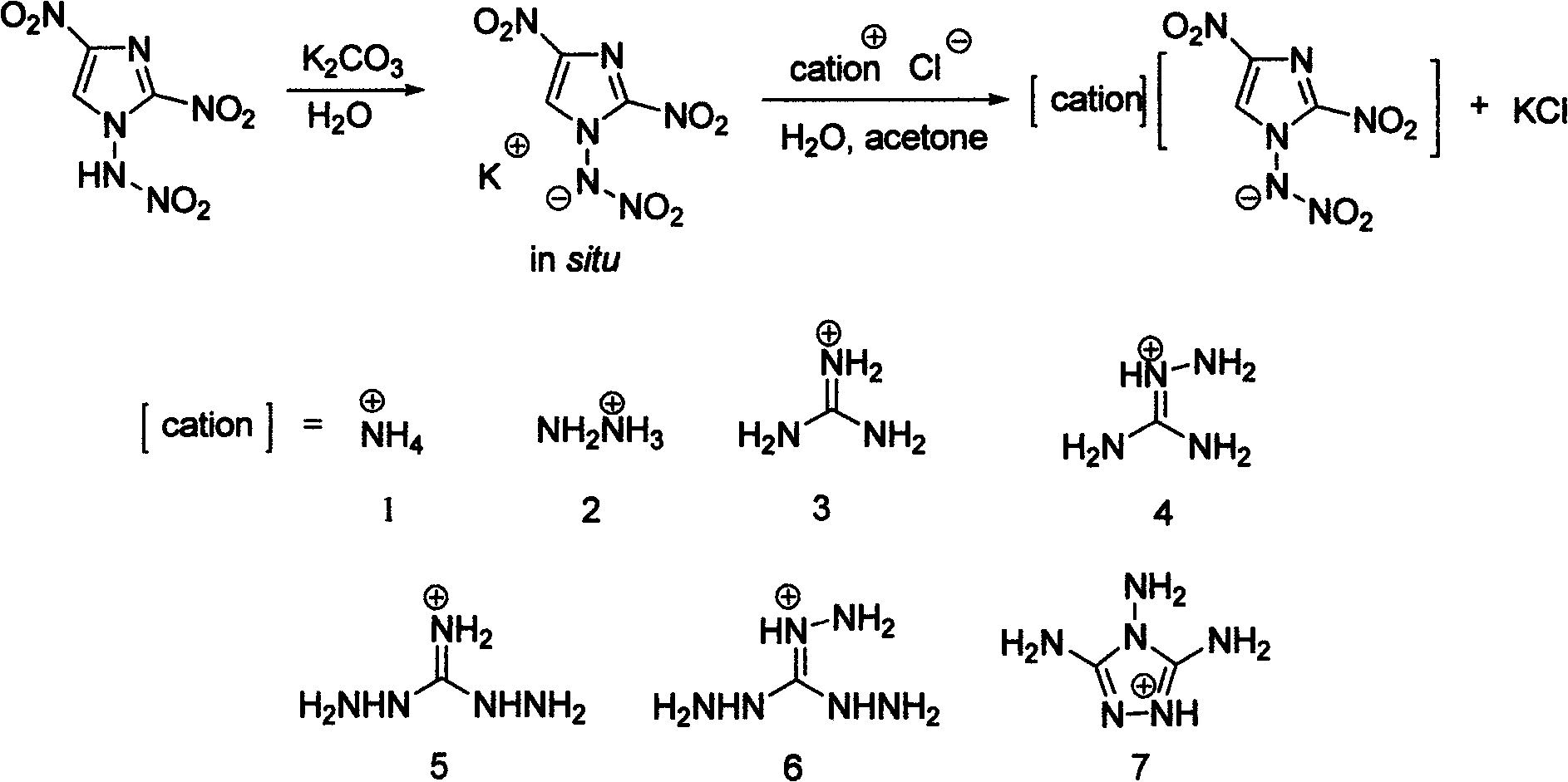
Energetic ion salts of 1-nitramine-2, 4-dimetridazloe and preparation method thereof
A technology of dinitroimidazole potassium salt and dinitroimidazole, which is applied in the field of 1-nitroamine-2, can solve the problems that the energy needs to be further improved, and achieve the effects of high yield, mild conditions and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 218mg (1.0mmol) 1-nitroamine-2,4-dinitroimidazole and 5mL deionized water into a 25mL single-necked flask to dissolve into a light yellow clear liquid, then add 69mg (0.5mmol) Potassium carbonate was stirred at room temperature for 10 minutes to generate carbon dioxide bubbles and simultaneously generate 1-nitroamine-2,4-dinitroimidazole potassium salt solution. Then, 53.5 mg of ammonium chloride was added to the reaction liquid, and the reaction was stirred at room temperature, and an off-white solid precipitate was precipitated. The reaction mixture was stirred at room temperature for 1 h, and the off-white precipitate was filtered, and the crude solid product was dried in a desiccator at room temperature. The crude product was recrystallized from a mixed solvent of 3 mL of acetone and 30 mL of diethyl ether to precipitate a solid, filtered and washed 3 times with diethyl ether, and dried at room temperature to obtain 212 mg of the product 1-nitroamine-2,4-dinitroi...
Embodiment 2
[0034] The conditions are the same as in Example 1, except that ammonia water is changed to hydrazine hydrate, and the product 2-(dinitromethyl)-3-nitro-1,3-diazacyclopent-1-ene hydrazine salt yield is 246mg, producing The rate is 98%.
[0035] Its structural formula is as follows:
[0036]
[0037] Decomposition temperature: 183°C (DSC). Density is 1.93g cm -3. 1 H NMR (400MHz, d 6 -DMSO): δ = 7.08 (s, 4H), 8.62 (s, 1H) ppm; 13 C NMR (100MHz, d 6 -DMSO): δ=123.16, 141.50ppm; IR (neat): 3190, 3142, 1555, 1492, 1431, 1367, 1337, 1290, 1149, 1024, 892, 820, 753, 632cm -1 ;elemental analysis(%) calcd for C 3 h 6 N 8 o 6 : C 14.42, H 2.42, N 44.80; found: C 14.39, H 2.40, N 44.69.
Embodiment 3
[0039] Condition is the same as embodiment 1, only changes ammoniacal liquor into the guanidine dissolved in a small amount of methanol, product 2-(dinitromethyl)-3-nitro-1,3-diazacyclopent-1-ene guanidine salt output is 267 mg, 96% yield.
[0040] Its structural formula is as follows:
[0041]
[0042] Decomposition temperature: 191°C (DSC). Density is 1.77g cm -3 . 1 H NMR (400MHz, d 6 -DMSO): δ=6.95 (t, 2H), 8.69 (s, 6H) ppm; 13 C NMR (100MHz, d 6 -DMSO): δ=123.61, 139.98, 157.92ppm; IR (neat): 3351, 3272, 3203, 3132, 1648, 1547, 1484, 1416, 1379, 1300, 1150, 988, 886, 818, 753, 634cm -1 ;elemental analysis(%) calcd for C 4 h 7 N 9 o 6 : C 17.33, H 2.55, N 45.48; found: C 17.27, H 2.51, N 45.33.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com