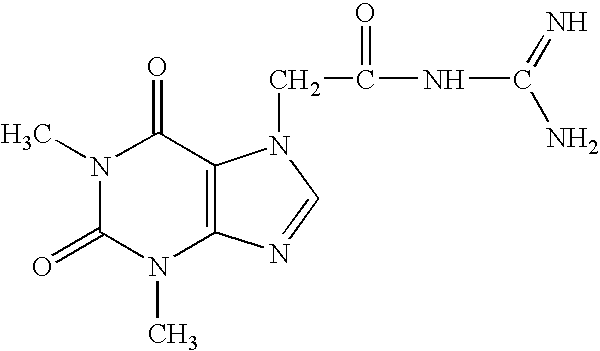
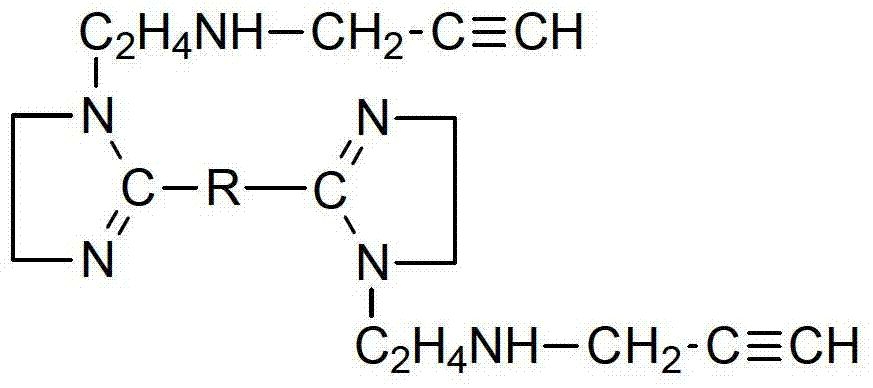
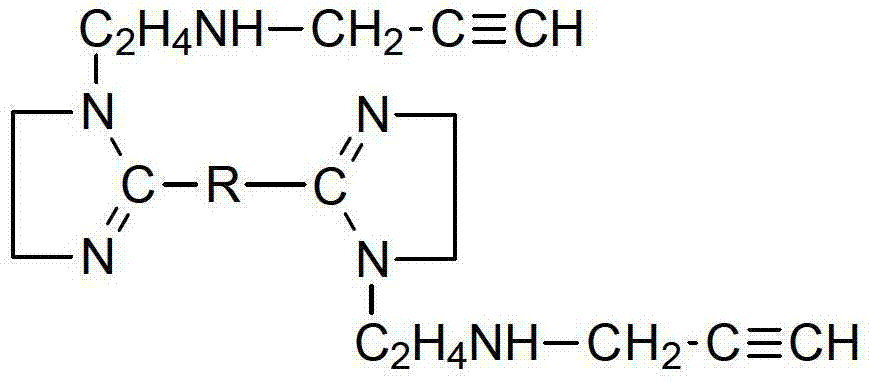
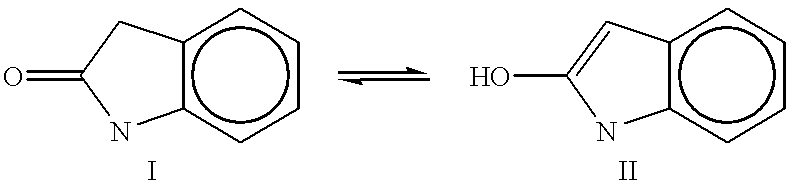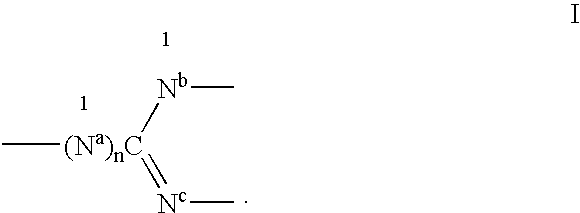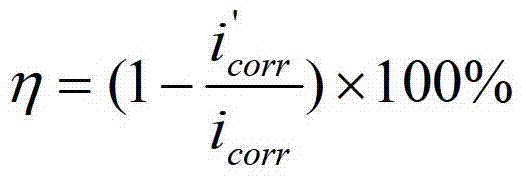Patents
Literature
60 results about "2-Imidazoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
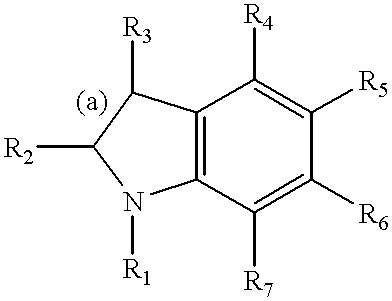
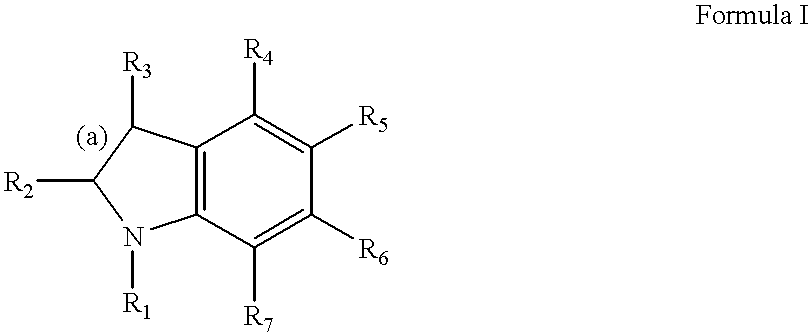
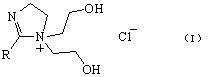
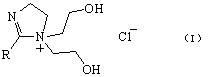
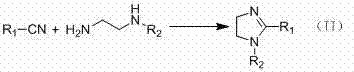
2-Imidazoline (dihydroimidazoles) is one of three isomers of the nitrogen-containing heterocycle imidazoline, with the formula C₃H₆N₂. The 2-imidazolines are the most common imidazolines commercially, as the ring exists in some natural products and some pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.
6-(2-imidazolinylamino)quinoxaline compounds useful as alpha-2 adrenoceptor agonists
InactiveUS6117871ANo adverse side effectsSubstantial activityBiocideAnimal repellantsQuinoxalineThiol
The subject invention relates to methods of treating alpha-2 adenoreceptor modulated disorders, comprising administration, to a mammal in need of such treatment, of a safe and effective amount of a compound having the following structure: wherein: (a) R is unsubstituted C1-C3 alkanyl or alkenyl; and (b) R' is selected from hydrogen; unsubstituted C1-C3 alkanyl or alkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thiol; and halo. The subject invention also relates compounds and compositions for preventing or treating of disorders modulated by alpha-2 adrenoreceptors.
Owner:THE PROCTER & GAMBLE COMPANY
2-imidazolinylaminoindole compounds useful as alpha-2 adrenoceptor agonists
This invention involves compounds having the following structure: wherein: a) R1 is hydrogen; or alkyl; bond (a) is a single or a double bond; b) R2 and R3 are each independently selected from hydrogen; unsubstituted C1-C3 alkanyl, alkenyl or alkynyl; cycloalkanyl, cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio; nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino and halo; c) R4, R5 and R6 are each independently selected from hydrogen; unsubstituted C1-C3 alkanyl, alkenyl or alkynyl; cycloalkanyl, cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio; nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino; halo; and 2-imidazolinylamino; and wherein one and only one of R4, R5 and R6 is 2-imidazolinylamino; d) R7 is selected from hydrogen; unsubstituted C1-C3 alkanyl, alkenyl or alkynyl; cycloalkanyl, cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio; nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino and halo; e) the compound is not 4-(2-imidazolinylamino)indole; enantiomers, optical isomers, stereoisomers, diastereomers, tautomers, addition salts, biohydrolyzable amides and esters thereof, and pharmaceutical compositions comprising such novel compounds. The invention also relates to the use of such compounds for treating disorders modulated by alpha-2 adrenoceptors.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Production of cationic microsphere with crosslinked swelling function
InactiveCN101029109AEasy speed controlControllable swelling ratioDrilling compositionCross-linkPersulfate
Production of cationic micro-sphere with cross-linking swelling function is carried out by taking persulfate and sulfite or persulfate and azo-diisobutyl nitrile binary composite or persulfate, sulfite and 2,2'-azo-(2-(2-imidazoline-2-radical)propane)dihydrochloride(VA-044)ternary composite as initiating agent, adding into dispersant and cross-linking agent, initiating DMC monomer and AM monomer in mixed medium of cyclohexane-water or industrial white oil-water or 120#solvent oil-water and reverse suspension polymerizing to obtain the final product. The grain size is 1-50 mu m, it has controllable swelling speed ratio and can be used for third oil-extraction plugging materials.
Owner:JIANGNAN UNIV
2-imidazolinylaminobenzoxazole compounds useful as alpha-2 adrenoceptor agonists
InactiveUS6110952AHigh activityNo adverse side effectsBiocideSenses disorderAdrenergicAlpha 2 adrenoceptors
Owner:THE PROCTER & GAMBLE COMPANY
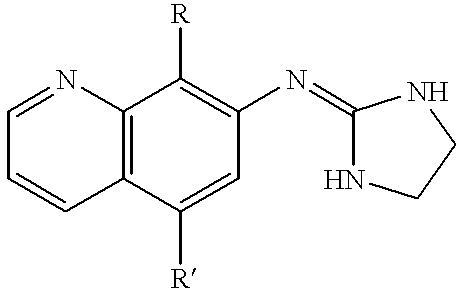
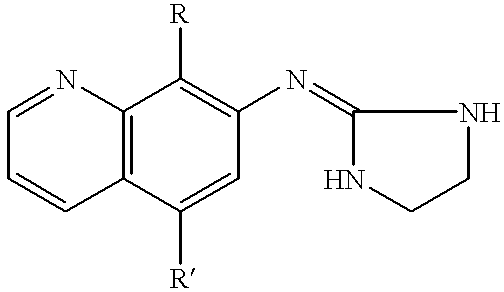
7-(2-imidazolinylamino) quinoline compounds useful as alpha-2 adrenoceptor agonists
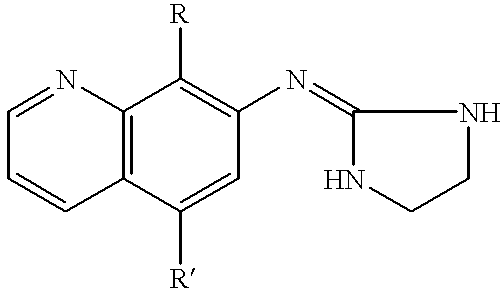
This invention involves involves the use of compounds having the following structure:wherein:(a) R is unsubstituted C1-C3 alkanyl or alkenyl; and(b) R' is selected from hydrogen; unsubstituted C1-C3 alkanyl or alkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thiol; cyano; and halo;for preventing or treating of disorders modulated by alpha-2 adrenoceptors.The subject invention also involves novel compounds and compositions.
Owner:THE PROCTER & GAMBLE COMPANY
Production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody
InactiveCN1569840AEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting food2-ImidazolineImidacloprid
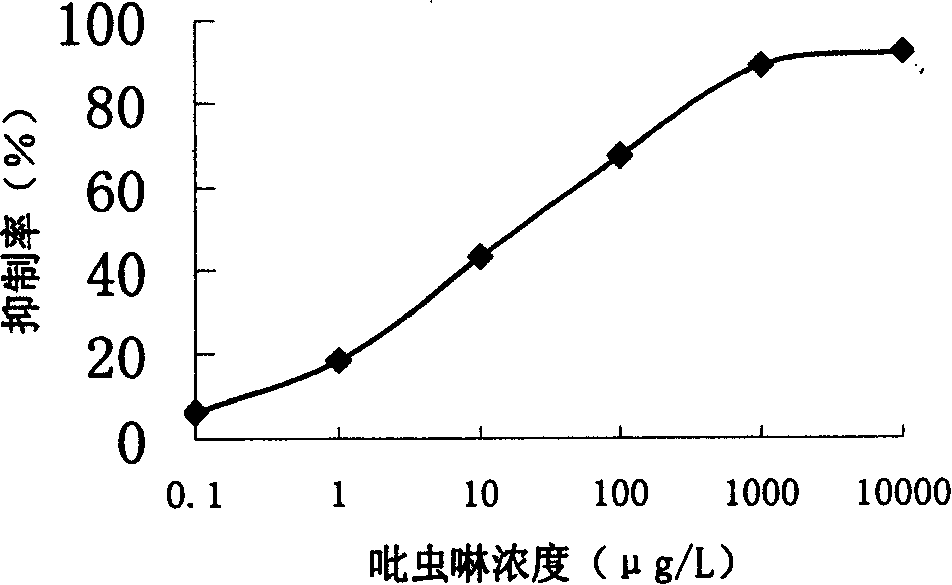
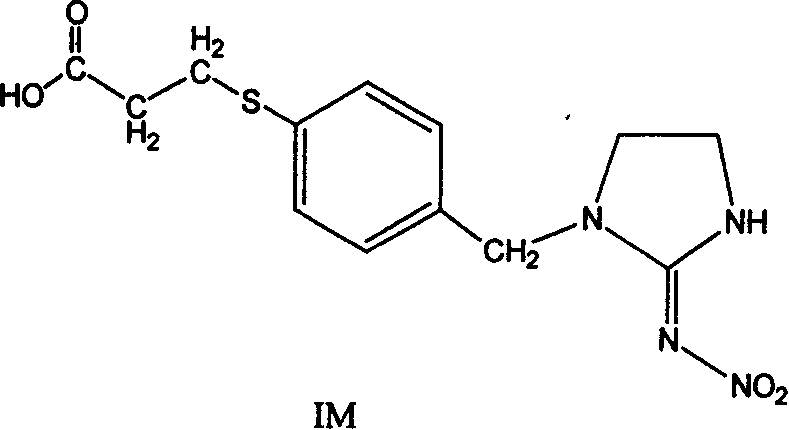
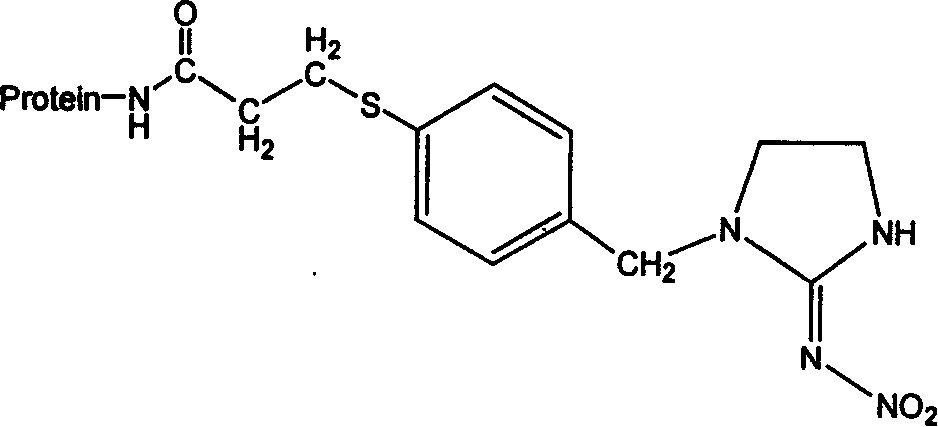
The invention discloses the production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody, wherein the production method comprises, using imidacloprid (1-(6-chlorine-3-picolyl)-N-nitro-2-imidazoline imine) as raw material for reaction with 3-mercaptopropionic acid under alkaline condition, thus synthesizing hapten 1-(6-(2-carboxyethyl) sulfo-3-picolyl)-N-nitro-2-imidazoline imines (IM), then coupling with proteins through carbodiimide method and mixed anhydride method to prepare artificial antigens (immunogens and peridium antigens).
Owner:ZHEJIANG UNIV
Water-resistance polyester powder coating
InactiveCN105062329AImprove water resistanceImprove heat resistanceAntifouling/underwater paintsPaints with biocidesPolyvinyl butyralEpoxy
The invention discloses water-resistance polyester powder coating. The powder coating is prepared from the following raw materials in parts by weight: 50-65 parts of pure polyester resin, 5-10 parts of fluorine-containing polyester resin, 3-10 parts of epoxy resin, 5-15 parts of organic silicon resin, 3-10 parts of carboxyl-terminated nitrile rubber, 1-5 parts of N,N,N',N'-tetrakis(2-hydroxyethyl)adipamide, 3-12 parts of tetrahydro-1,3,4,6-tetrakis(methoxymethyl)imidazo[4,5-d]imidazole-2,5(1H,3H)-dione, 1-5 parts of 2-phenyl-2-imidazoline, 1-5 parts of 2-methylimidazole, 2-5 parts of polyethylene, 3-10 parts of polyvinyl butyral, 10-25 parts of pigment and 2-10 parts of auxiliaries. The water-resistance polyester powder coating has good water resistance and good oil resistance, excellent acid and alkali corrosion resistance, can be used for outdoor coating, and has the advantages of good comprehensive performance and long service life.
Owner:安徽圣德建材科技有限公司
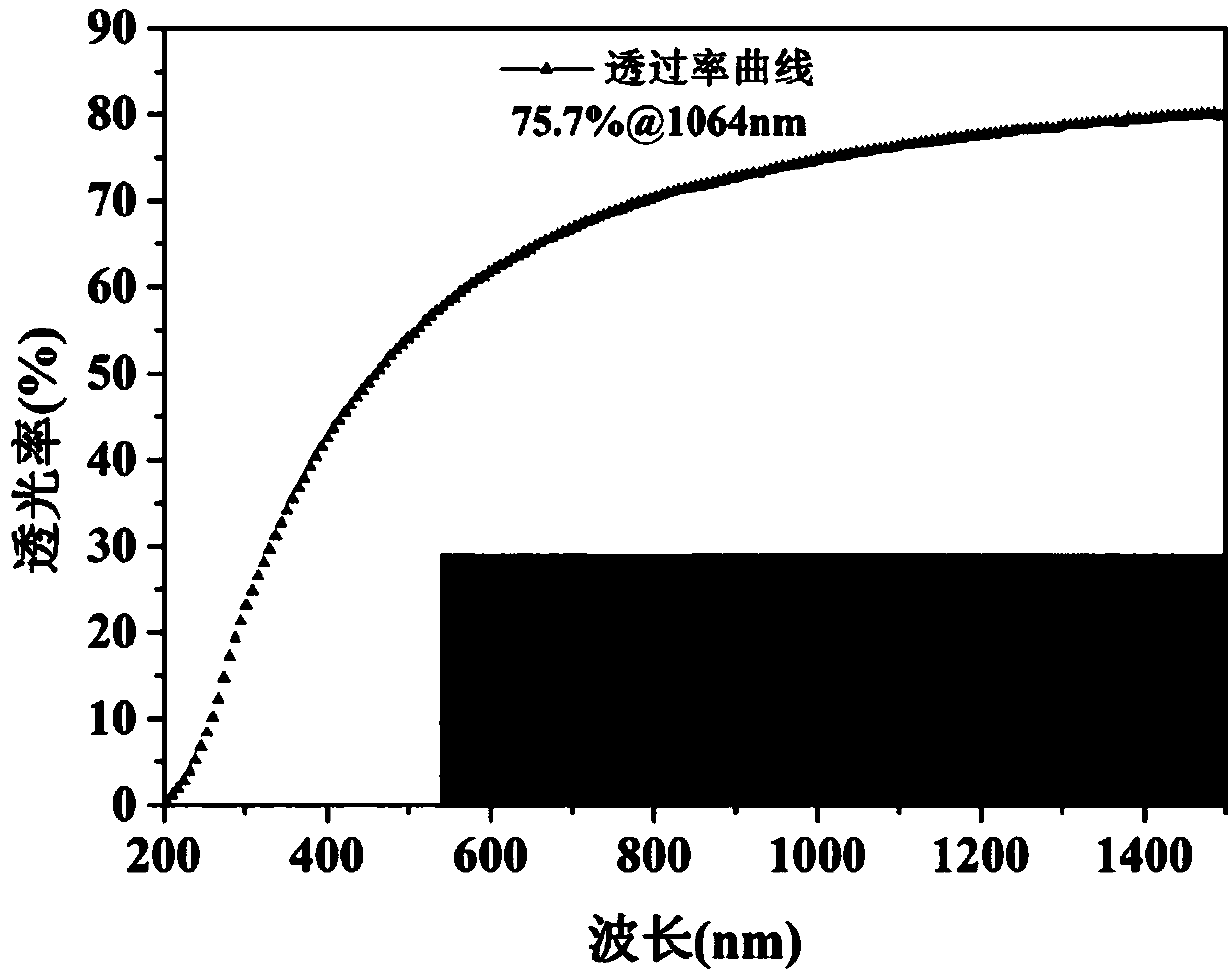
Method for preparing YAG transparent ceramic by gel casting
ActiveCN107721424AIncrease the rate of gelationImprove gel forming efficiencyZiegler–Natta catalyst2-Imidazoline
The invention belongs to the technical field of molding and manufacturing processes for transparent ceramic, and specifically relates to a method for preparing YAG transparent ceramic by gel casting.The method uses Isobam as a dispersing agent and a gelating agent, and adopts a system selected from the group consisting of a Ziegler-Natta catalyst system (a Ziegler-Natta catalyst), an ammonium persulfate-tetramethylethylenediamine (APS-TEMED) catalytic system and a 2,2-azo[2-(2-imidazoline-2-yl)propane]hydrochloride (AZIP.2HCl) catalytic system to control the rate of gelation and control the time of gel casting.
Owner:XUZHOU NORMAL UNIVERSITY
High-performance thermosetting polyester powder coating
ActiveCN105062330AImprove water resistanceImprove heat resistanceAntifouling/underwater paintsPaints with biocides2-ImidazolineCorrosion
The discloses a high-performance thermosetting polyester powder coating which comprises the raw materials: pure polyester resin, fluorinated polyester resin, epoxy resin, terminal carboxyl nitrile rubber, N,N,N,N'-tetra(2-ethoxyl) adipamide, tetramethoxyl methyl glycoluril, triglycidyl isocyanurate, amino resin, phenyl-2-imidazoline, 2-methylimidazole, chitosan, sodium lignin sulfonate, starch, polyethylene, polyvinyl butyral, nano titanium dioxide, aluminium oxide, zinc borate, mica iron oxide, montmorillonite, nanosilver, activated carbon powder, lanthanum oxide and auxiliaries. The high-performance thermosetting polyester powder coating disclosed by the invention is good in water resistance, oil resistance and heat resistance and excellent in weather resistance, acid and alkaline corrosion resistance and pollution resistance, and is good in comprehensive performance and long in service life if being used as an outdoor coating.
Owner:黄山佳杰新材料科技有限公司
2-imidazolinylaminoindole compounds useful as alpha-2 adrenoceptor agnonists
InactiveUS6395764B1No adverse side effectsSubstantial activityBiocideSenses disorderAdrenergicEnantiomer
This invention involves compounds having the following structure:wherein:a) R1 is hydrogen; or alkyl; bond (a) is a single or a double bond;b) R2 and R3 are each independently selected from hydrogen; unsubstituted C1-C3 alkanyl, alkenyl or alkynyl; cycloalkanyl, cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio; nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino and halo;c) R4, R5 and R6 are each independently selected from hydrogen; unsubstituted C1-C3 alkanyl, alkenyl or alkynyl; cycloalkanyl, cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio; nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino; halo; and 2-imidazolinylamino; and wherein one and only one of R4, R5 and R6 is 2-imidazolinylamino;d) R7 is selected from hydrogen; unsubstituted C1-C3 alkanyl, alkenyl or alkynyl; cycloalkanyl, cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio; nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino and halo;e) the compound is not 4-(2-imidazolinylamino)indole;enantiomers, optical isomers, stereoisomers, diastereomers, tautomers, addition salts, biohydrolyzable amides and esters thereof, as well as pharmaceutical compositions comprising such novel compounds. The invention also relates to the use of such compounds for preventing or treating disorders modulated by alpha-2 adrenoceptors.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Monoclonal antibody for detecting imidacloprid pesticide residue
InactiveCN101880325AHigh analytical capacityLarge analysis capacitySerum albuminMicroorganism based processesBALB/cPesticide residue
The invention relates to an imidacloprid pesticide against monoclonal antibody and a preparation method thereof, and belongs to the technical field of biology. The preparation method comprises the following steps of: immunizing a BALB / c mouse by using a coupling substance of immune hapten 1-[6-(2-carboxyethylsulfenyl-3-pyridine) methyl]-N-nitro-2-imidazoline imine and bovine serum albumin, preparing hybrid tumor cells from spleen cells and myeloma cells Sp2 / 0 of the immunized mouse by the hybrid tumor technology, and obtaining hybrid tumor strains 2F11 / A9 capable of stably secreting the imidacloprid pesticide against monoclonal antibody. By effectiveness verification, the antibody can be used for sensitive and quick detection of imidacloprid residues in agricultural production environments and agricultural products. The preparation technique for the imidacloprid pesticide against monoclonal antibody is simple and feasible, does not need special instruments and equipment in the whole preparation process of the antibody, and is easy for scale production in factories.
Owner:NANJING AGRICULTURAL UNIVERSITY
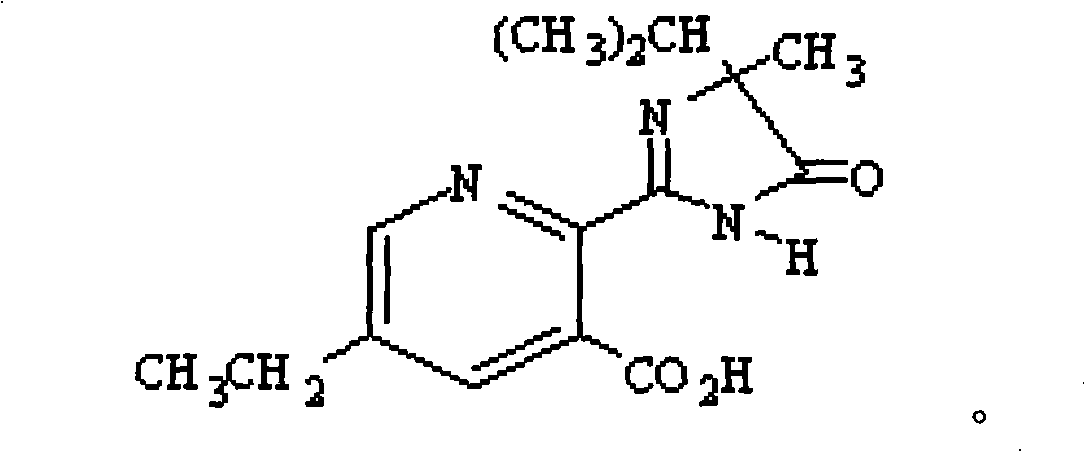
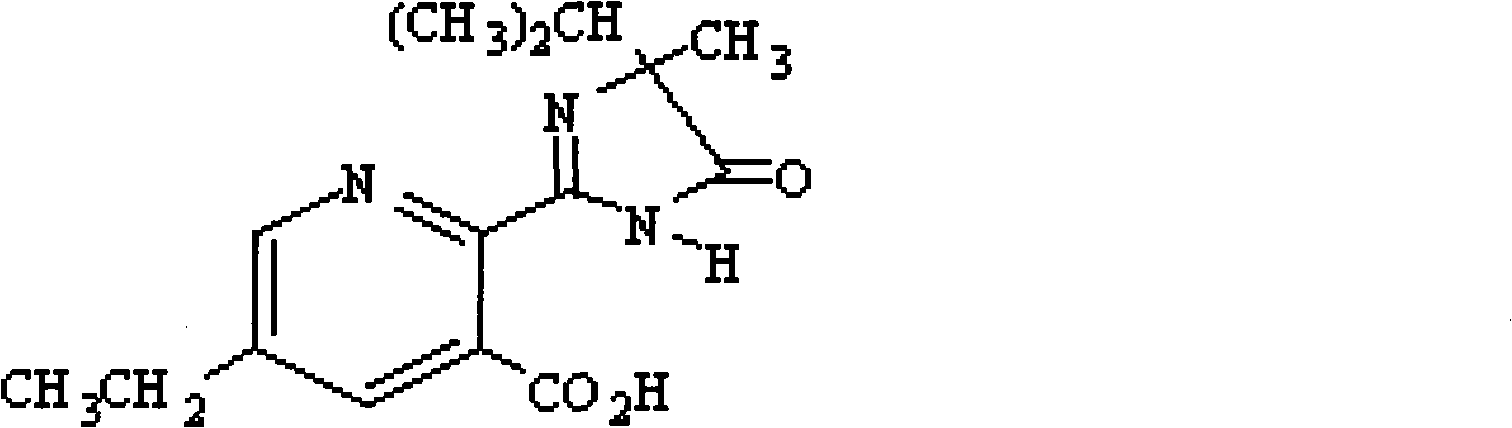
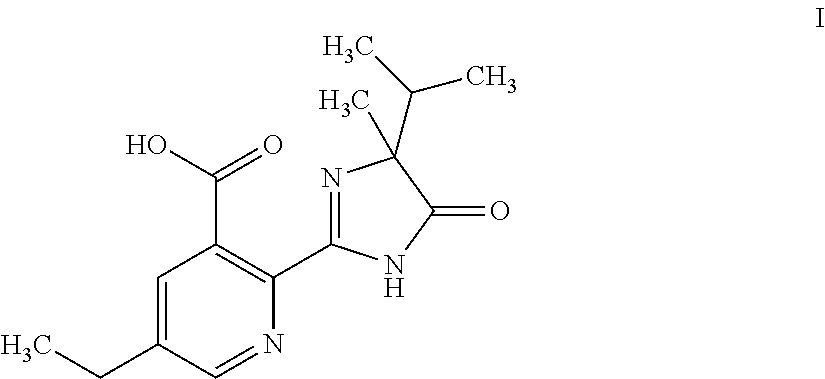
Uses of compound (RS)-5-ethyl-2-(4-isopropyl-4-methyl-5-oxo-2-glyoxalidine-2-yl)nicotinic acid and aminium salt thereof as plant chemical hybridizing agent
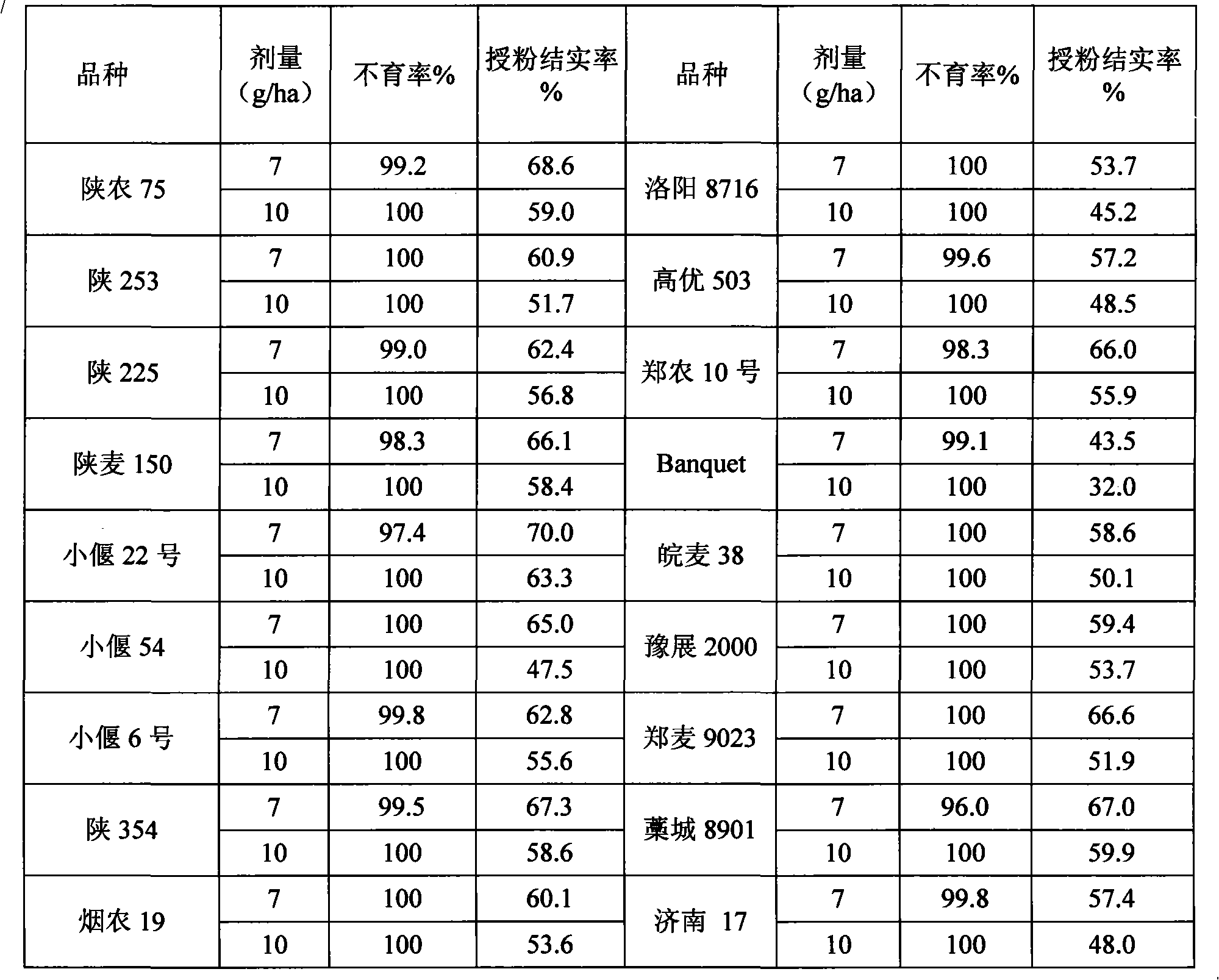
The invention discloses application of a known compound (RS)-5-ethyl-2-(4-isopropyl-4-methyl-5-oxo-2-imidazoline-2-yl) nicotinic acid and ammonium salt thereof used as phytochemistry hybridizing agents, wherein the compound (RS)-5-ethyl-2-(4-isopropyl-4-methyl-5-oxo-2-imidazoline-2-yl) nicotinic acid and the ammonium salt thereof are prepared into dosage forms such as a liquid preparation, a dry suspending agent, a water dispersible granule, emulsifiable solution, wettable powder or other suitable agricultural dosage forms which are diluted to prepare an aqueous solution for spraying leaf surfaces before use; and the dosage of effective active ingredients of the compounds is between 1 and 15 grams per hectare. Experiments prove that the two compounds can be used as highly-efficient, low-cost and low-toxicity chemical hybridizing agents for gramineae and brassica plants.
Owner:NORTHWEST A & F UNIV
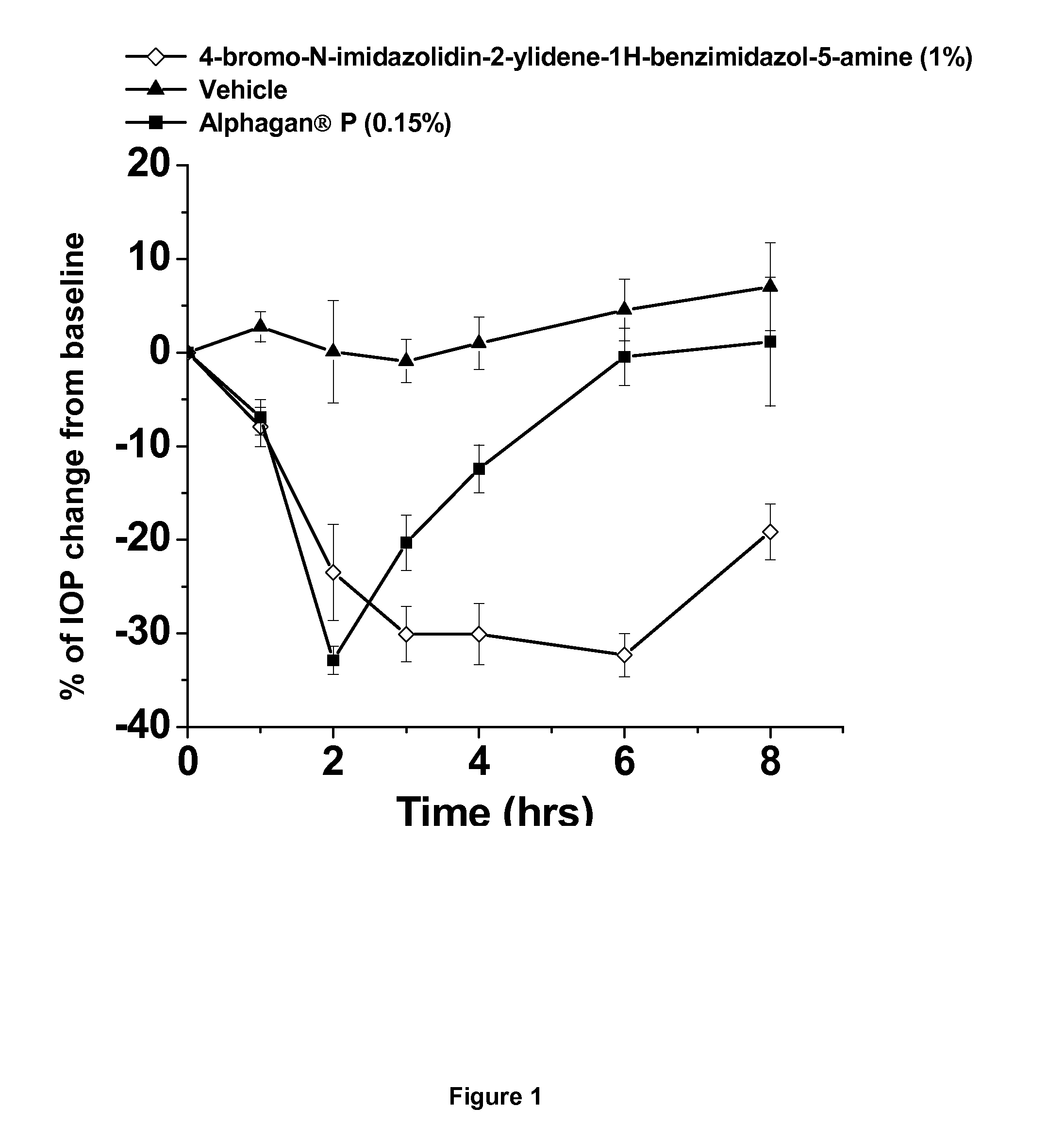
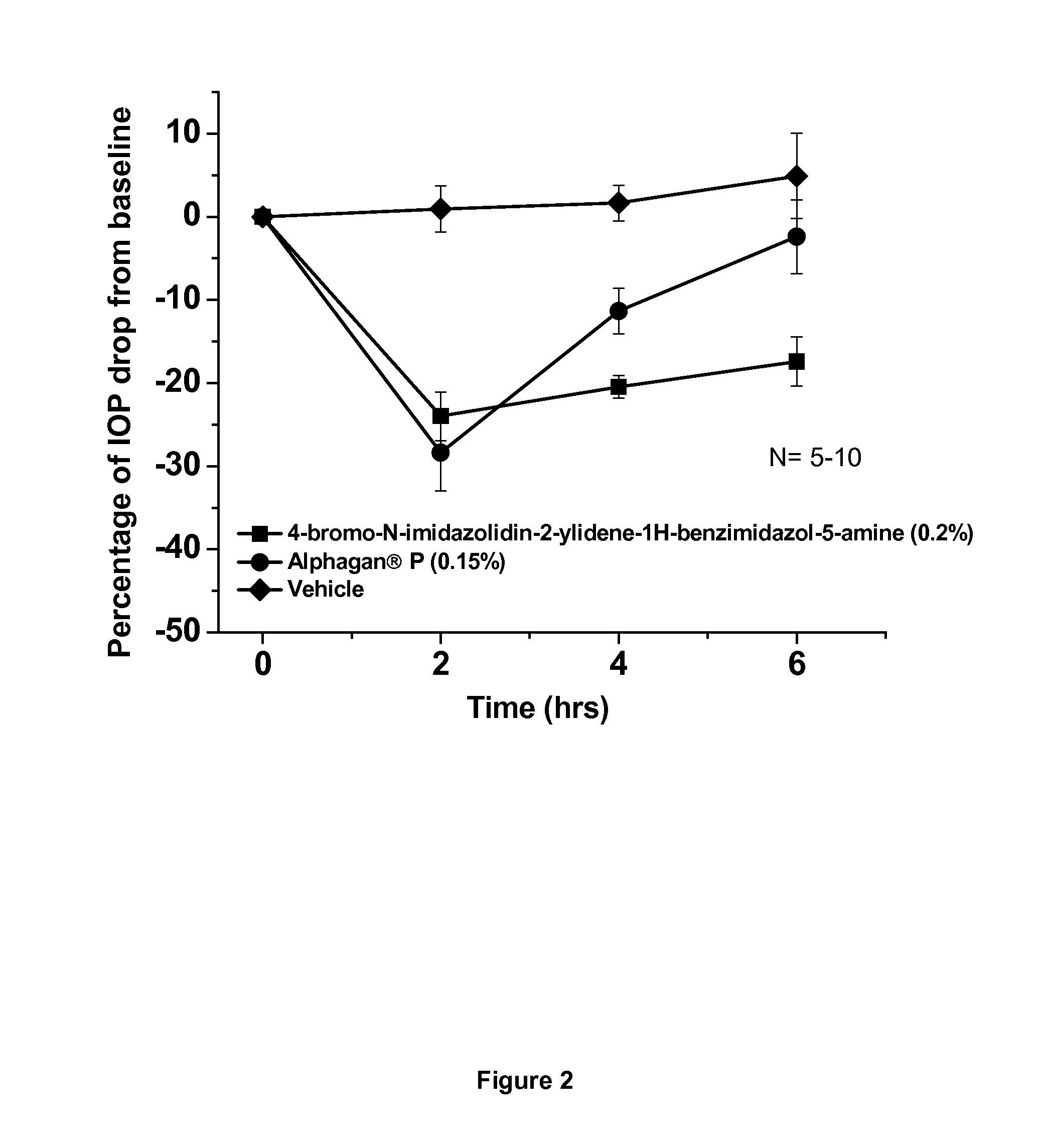
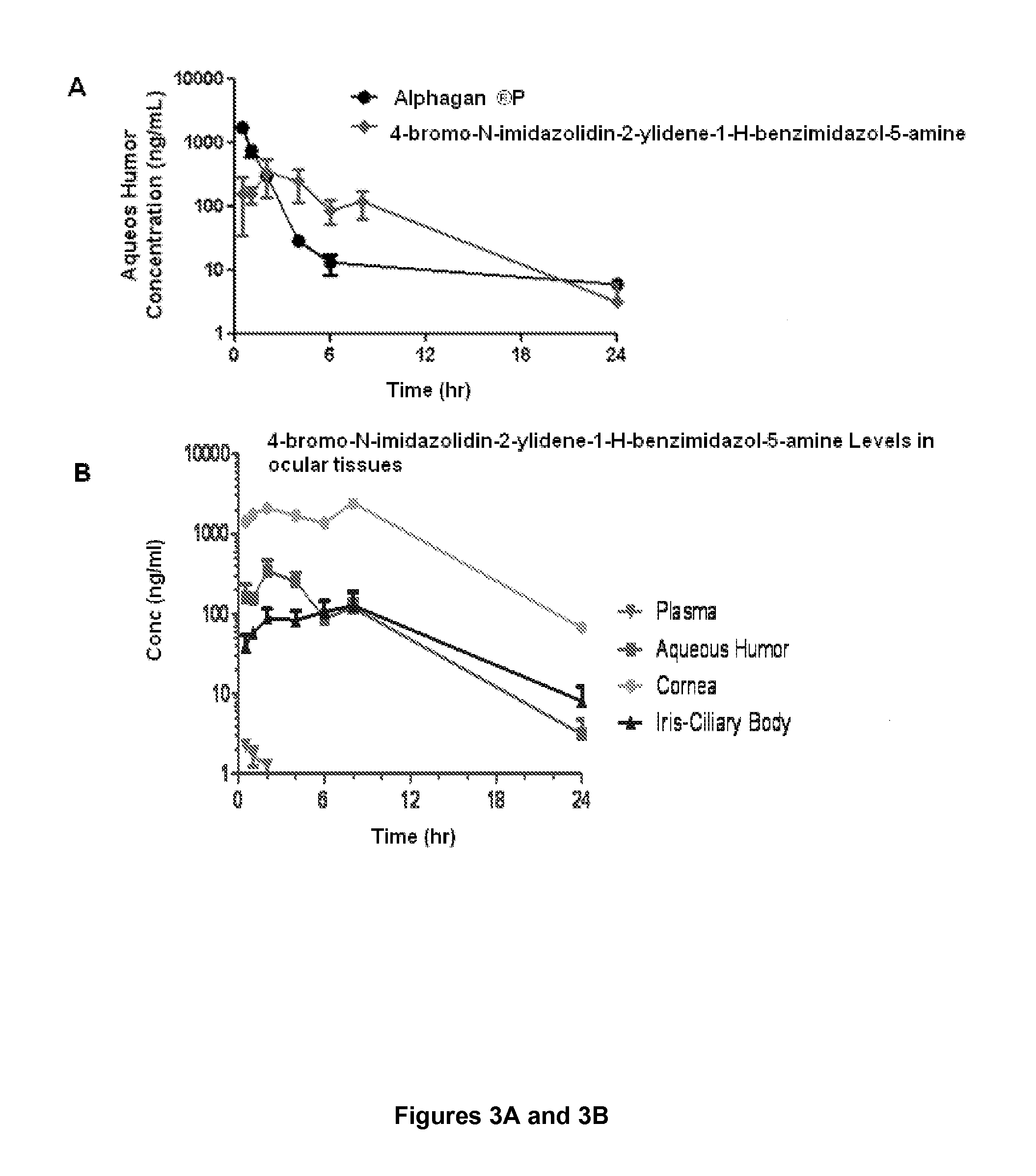
Alpha-2 adrenergic agonist having long duration ofintraocular pressure-lowering effect
ActiveUS20110178145A1Facilitated releaseLower eye pressureBiocideOrganic active ingredientsAdrenergic2-Imidazoline
The present invention provides a method of lowering intraocular pressure which comprises administering a therapeutically effective amount of a pharmaceutical composition comprising 4-bromo-5-(2-imidazolin-2-ylamino)benzimidazole, or a salt thereof to the affected eye of a patient, as a single dose, wherein the affected eye has an intraocular pressure less than the baseline intraocular pressure for at least eight (8) hours.
Owner:ALLERGAN INC
Nylon corrugated pipe
InactiveCN103554664AImprove high temperature resistanceHigh surface glossWear resistant2-Imidazoline
The invention discloses a nylon corrugated pipe which is characterized by being composed of the following raw materials in parts by weight: 93-100 parts of polypropylene T30S, 30-36 parts of nylon 610, 1-2 parts of methacrylic acid, 2-3 parts of dibutyl phthalate, 1-2 parts of aluminum tripolyphosphate, 0.8-1 part of methyltin mercaptide, 2-3 parts of tricalcium silicate, 1-2 parts of 2-phenyl-2-imidazoline, 1-3 parts of diisopropylethanolamine, 0.6-1 part of 2-hydroxy-4-n-octyloxybenzophenone, 1-2 parts of pine tar and 10-12 parts of composite filler. The corrugated pipe disclosed by the invention has favorable high temperature resistance, is particularly suitable for being used as a corrugated pipe material dried under a high temperature condition, and is wear-resistant, strong in chemical corrosion resistance, high in surface glossiness, long in service life, strong in impact resistance and high in hardness.
Owner:SINOMACH GENERAL MACHINERY SCIENCE & TECHNOLOGY CO LTD
Preparation and application of near-infrared-response photodynamic optothermal treatment nano-composite material
ActiveCN108939073AGood biocompatibilityStrong absorption capacityPhotodynamic therapyAntineoplastic agentsTreatment effectGeneration rate
The invention discloses preparation of a near-infrared-response photodynamic optothermal treatment nano-composite material. The preparation comprises the following steps: dispersing a nano-silicon dioxide carrier into a solvent, adding CuCl2.2H2O solution, stirring, and carrying out dispersion in ultrapure water; adding an Na2S.9H2O solution, stirring at room temperature, then stirring at 90 DEG C, and carrying out dispersion in ultrapure water, so as to obtain suspension liquid; and adding a temperature-sensitive photodynamic reagent into the suspension liquid, dissolving a phase-conversion material into absolute ethyl alcohol, adding absolute ethyl alcohol into the suspension liquid, stirring at the room temperature for 4-8 hours, carrying out centrifugal washing, and drying, so as to obtain the finished nano-composite material, wherein the solvent is ultrapure water or absolute ethyl alcohol, the temperature-sensitive photodynamic reagent is 2,2-aza-bis(2-imidazoline) dihydrochloride and the phase-conversion material is tetradecyl alcohol or lauric acid. When the nano-composite material is used, the infrared induced excitation temperature is 37-44 DEG C, and the time is 2-6 hours. The nano-composite material is good in biocompatibility, relatively strong in absorptivity in a near infrared region, relatively high in photo-thermal conversion rate and free radical generation rate, high in loading capacity, has a good treatment effect and does not have side reaction; and the process is simple, low in cost and wide in application range.
Owner:NANJING UNIV OF POSTS & TELECOMM
Method for synthesizing phenylacetic acid from benzyl chloride carbonyl
InactiveCN102050721ASolve the cumbersome recycling processEasy to handleOrganic-compounds/hydrides/coordination-complexes catalystsCarboxylic preparation from carbon monoxide reactionSolubilityPhenylacetic acid
The invention discloses a method for synthesizing phenylacetic acid from benzyl chloride carbonyl. Co(PPh3)2Cl2 is taken as a main catalyst, and 2-alkyl-1-di(2-ethoxyl)-2-imidazoline chloride which is shown as a structural formula (1) is taken as a phase transfer catalyst, wherein R is alkyl with 1 to 5 carbon atoms. The short-chain 2-alkyl-1-di(2-ethoxyl)-2-imidazoline chloride is taken as the phase transfer catalyst and has high biodegradability, low toxicity, enhanced water solubility, has a good corrosion inhibition effect on metal equipment, and is easy to separate compared with a long-chain phase transfer catalyst.
Owner:NORTHWEST UNIV
Neurologically active compounds and compounds with multiple activities
Owner:NAFTCHI N ERIC
Compound containing triple-bond imidazoline, carbon dioxide corrosion inhibitor containing triple-bond imidazoline, and preparation method of carbon dioxide corrosion inhibitor
ActiveCN104513204AImprove protectionGood water solubilityOrganic chemistryDrilling compositionSolubilityCarbon dioxide corrosion
The invention relates to a compound containing triple-bond imidazoline, a carbon dioxide corrosion inhibitor containing triple-bond imidazoline, and a preparation method of the carbon dioxide corrosion inhibitor. The carbon dioxide corrosion inhibitor comprises a compound containing triple-bond imidazoline, a non-ionic surfactant, and a solvent composed of alcohols with a low molecular weight. The corrosion inhibitor is used to protect oil well, gas well, and ground gathering pipelines in oil-gas field, and equipment and pipelines of a water treatment system from carbon dioxide corrosion. The triple-bond diimidazoline compound is prepared through the following steps: carrying out intramolecular dehydration reactions and intermolecular dehydration reactions between dicarboxylic acid and polyamine, and then making the reaction products carry out reactions with compounds containing triple-bonds. The triple-bond diimidazoline compound contains a plurality of active adsorption centers such as two five-membered diazaheterocycles, groups containing triple-bonds, and the like. The organic adsorption film formed by the compound has a better protective effect on carbon steel. The provided corrosion inhibitor has good water solubility, so that the corrosion inhibitor can be dissolved in oil-gas filed output liquid with a high mineralization degree without using a solvent composed of alcohols with a lower molecular weight. The carbon dioxide corrosion inhibitor has the advantages of good corrosion inhibiting performance, good compatibility, and high water solubility.
Owner:YANAN SHUANG FENG GRP
Rare earth aluminum electrode anti-corrosion paint and preparation method thereof
InactiveCN105860599AImprove corrosion resistanceEasy to operateAnti-corrosive paintsRare earth2-Imidazoline
Owner:ANHUI KAILIN ADVANCED MATERIAL CO LTD
2-(p-alkoxyphenyl)-2-imidazolines and their use as corrosion inhibitors
InactiveUS20160347988A1Suppresses anodic reactionPreventing and reducing corrosionDrilling compositionBorehole/well accessories2-ImidazolineChain length
An aminoalkyl imidazolines of the formula:having p-octyloxy-, p-dodecyloxy-, or p-octadecyloxy-phenyl pendants as hydrophobes, for use to mitigate mild steel corrosion. An electron-rich aromatic ring, in conjugation with an amidine motif, imparts increasing corrosion inhibition efficiencies with an increasing hydrophobe chain length. X-ray photoelectron spectroscopy confirms the formation of an aminoalkyl imidazoline film on a metal surface prior to reaching a critical molar concentration.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
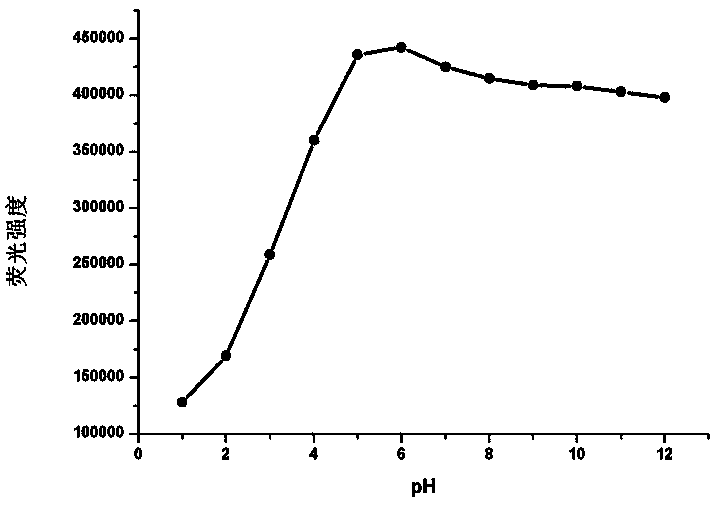
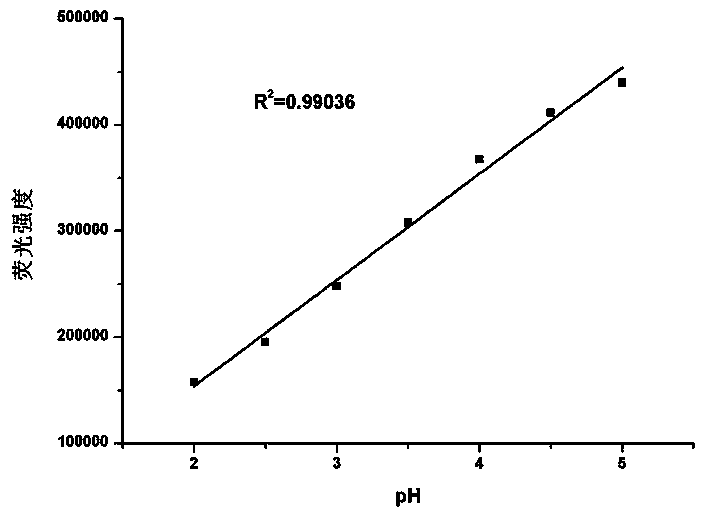
Application of methyl-2-imidazoline as pH value and Fe<3+> dual-functional fluorescent probe and fluorescent probe prepared from methyl-2-imidazoline
InactiveCN107629786ASmall side effectsGood choiceFluorescence/phosphorescenceLuminescent compositionsSolubilitySide effect
The invention relates to the application field of a pH probe and metal ion recognition, in particular to an application of methyl-2-imidazoline as a pH value and Fe<3+> dual-functional fluorescent probe and the dual-functional fluorescent probe prepared from methyl-2-imidazoline and used for pH sensing and Fe<3+> detection. The fluorescent probe has the benefits as follows: the fluorescent substance methyl-2-imidazoline has small toxic or side effects, good selectivity and high sensitivity, is firstly found to have good fluorescence characteristics and is applied to the field of fluorescent probes. The synthesis mechanism of a fluorescent compound is manure, the fluorescent compound is low in cost and high in water solubility, and measurement of acidic pH (2.0-5.0) of a water solution as well as recognition and quantitative detection of Fe<3+> in the physiological environment can be realized.
Owner:UNIV OF JINAN
A kind of environment-friendly steel surface antirust agent and preparation method thereof
Owner:珠海大利刀具有限公司
Preparation method for 4-pyridinecarboxaldehyde
InactiveCN106518753AWide variety of sourcesLow priceOrganic chemistryEthylenediamineProduction effect
The invention discloses a preparation method for 4-pyridinecarboxaldehyde. The preparation method specifically comprises the following steps that firstly, a solvent-free reaction is conducted, specifically, isonicotinic acid and ethylenediamine or o-diaminobenzene are reacted under the solvent-free condition to synthesize 4-pyridine-2-imidazoline; and secondly, reductive hydrolysis is conducted, specifically, the synthesize 4-pyridine-2-imidazoline is subjected to reductive hydrolysis to synthesize the 4-pyridinecarboxaldehyde. The method is wide in raw material source, low in raw material cost and production cost, simple in preparation process, suitable for large-scale industrial production, little in by-product, short in preparation time, high in yield and good in production effect.
Owner:安徽省诚联医药科技有限公司
Preparation method and application of 2-phenyl-2-imidazoline and pyromellitic dianhydride adduct
InactiveCN109824500AReduce glossPreparation from carboxylic acid anhydridesPowdery paintsCycloneEpoxy
The invention relates to a preparation method of 2-phenyl-2-imidazoline and pyromellitic dianhydride adduct. The preparation method comprises the steps of (1) synthesizing 2-phenyl-2-imidazoline; (2)dropwise adding the 2-phenyl-2-imidazoline of the step (1) into pyromellitic dianhydride solution or suspension emulsion in equal molar ratio, allowing them to react to generate a salt adduct, treating with a centrifuge, drying by cyclone, and collecting with a trap to obtain the 2-phenyl-2-imidazoline and pyromellitic dianhydride adduct. The preparation method has the advantages that the final product has organic amine functional groups and carboxylic functional groups; the product has the catalytic curing function of imidazole and the reacting function of carboxylic acid, is mainly applied to the field of matte powder paints to mainly function to eliminate gloss, allows a coating to gain very low gloss and evenly zero gloss, which is hard for common materials to achieve, may also act asan epoxy resin curing agent and is also applicable as epoxy curing agents or catalysts in other fields.
Owner:阜阳市诗雅涤新材料科技有限公司
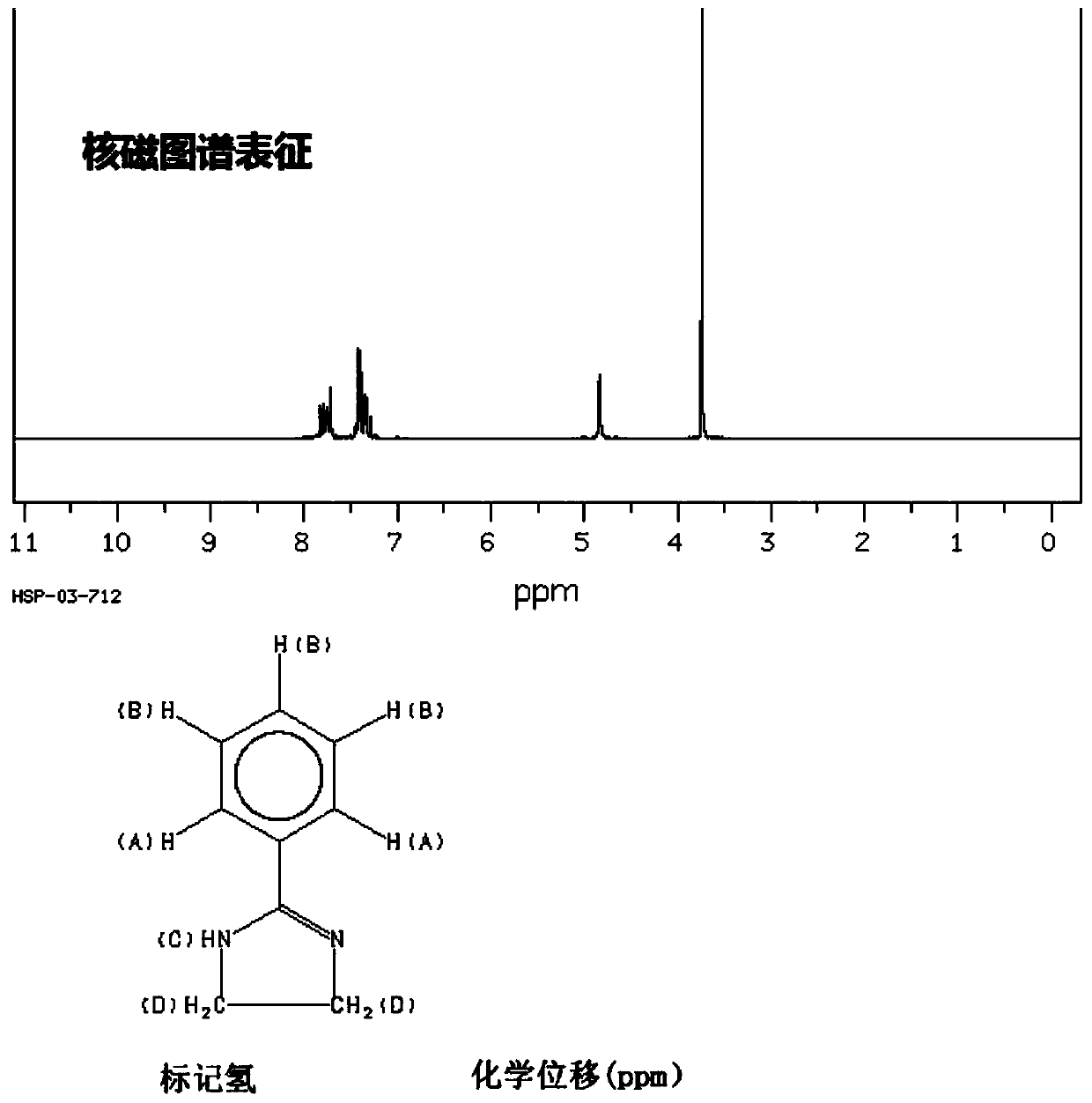
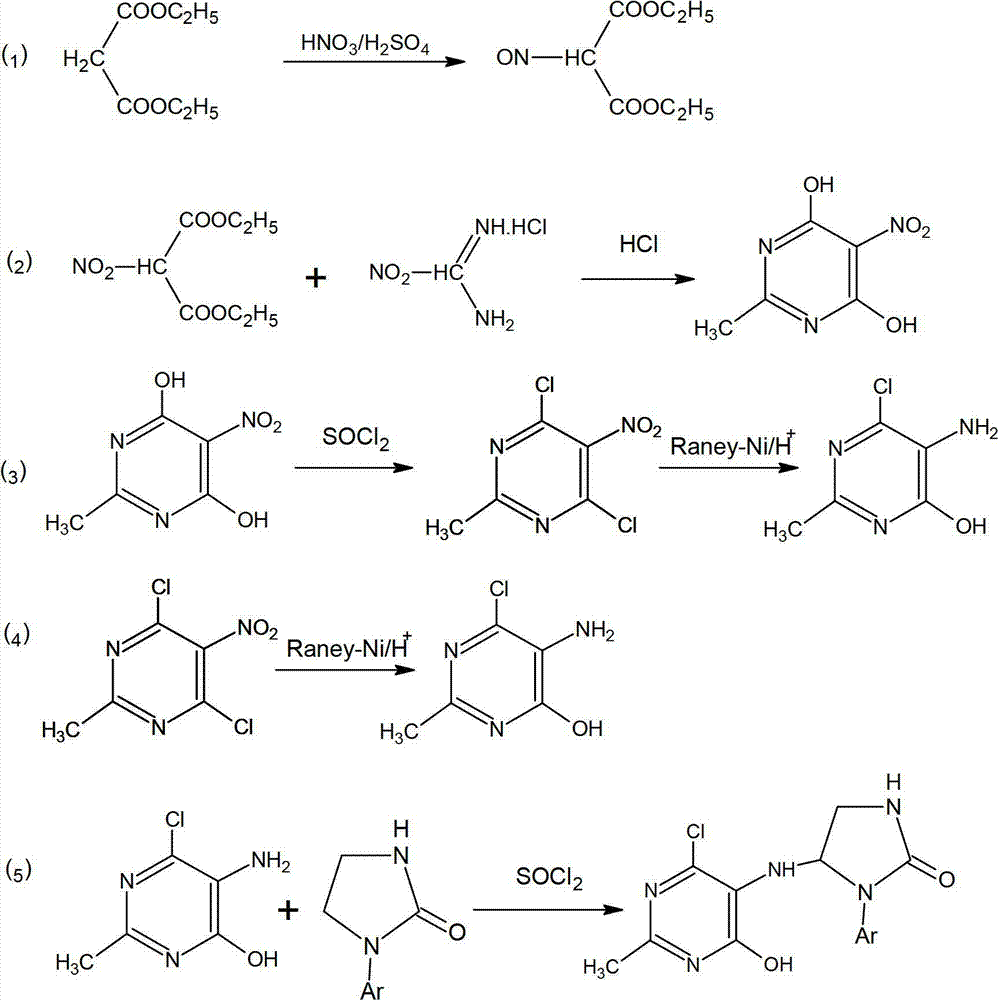
4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine preparation method
The invention discloses a 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine preparation method. The preparation method includes: firstly, feeding concentrated nitric acid and concentrated sulfuric acid into diethyl malonate for nitrifying diethyl malonate to obtain diethyl 2-nitromalonate; secondly, enabling the diethyl 2-nitromalonate and acetamidine hydrochloride to cyclize to obtain 2-methyl-5-nitro-4, 6-dihydroxypyrimidine under the action of 12N hydrochloric acid; thirdly, chloridizing the 2-methyl-5-nitro-4, 6-dihydroxypyrimidine with sulfoxide chloride serving as a chlorinating agent and hydrogenating and reducing the chloridized 2-methyl-5-nitro-4, 6-dihydroxypyrimidine with Raney nickel; and finally, condensing a product obtained in the third step and acetyl imidazo-2-one, so that 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine is obtained. The preparation method is short in reaction time, higher than 80% in yield and simple and convenient to operate as reaction processes are carried out under the normal pressure, the 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine is low in production cost, phosphorus wastewater is avoided, and emission of waste gases, wastewater and industrial residues is low.
Owner:上海旭东海普南通药业有限公司
Process for the preparation of irbesartan hydrochloride
The present invention is concerned with a process for the preparation of 2n-butyl-4-spirocyclopentane-1-[(2′-(tetrazol-5-yl)biphenyl-4-yl)methyl]-2-imidazolin-5-one hydrochloride, irbesartan hydrochloride, novel hydrated and anhydrous crystalline forms thereof, amorphous irbesartan hydrochloride, formulations containing the same, therapeutic uses thereof and methods of treatment employing the same. The process of the present invention is a one-pot process which comprises reacting intermediate compounds 2n-butyl-1,3-diazaspiro[4,4]non-1-en-4-one and 5-(4′-bromomethyl-biphenyl-2-yl)-1-trityl-1H-tetrazole.
Owner:CIPLA LTD
Leather sofa detergent added with sapindus saponin
InactiveCN105296246AEfficient removalLess corrosiveSurface-active non-soap compounds and soap mixture detergentsLeather surface finishingSulfonateSodium ricinoleate
The invention discloses a leather sofa detergent added with sapindus saponin. The leather sofa detergent is prepared from, by weight, 12-18 parts of fatty methyl ester sulfonates, 10-15 parts of sapindus saponin, 4-7 parts of sodium ricinoleate soap, 5-8 parts of rhamnolipid, 4-6 parts of sodium carbonate, 5-10 parts of sodium tripolyphosphate, 8-12 parts of tall oil, 14-22 parts of cocoamido propyl hydroxy sulfobetaine, 4-6 parts of 1,3-dimethyl-2-imidazolone, 5-10 parts of dodecyl dimethyl benzyl ammonium chloride, 7-11 parts of secondary alkyl sodium sulfonate, 10-15 parts of N-polyoxyethylated-N-tallow-alkylamine and 70-80 parts of water. The leather sofa detergent can effectively remove dirt, further has the bactericidal, antibacterial, environmentally friendly, pollution-free, small-leather-corrosivity advantages and meanwhile can improve the softness of leather sofa.
Owner:MINGGUANG LIANGYU FURNITURE CO LTD
Herbicidal mixtures comprising imazethapyr, imazamox and fomesafen, sulfentrazone or bentazone
The present invention relates to ternary herbicidally active compositions, which comprise a) 5-ethyl-2-[(RS)-4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl]nicotinic acid (common name: imazethapyr) and 2-[(RS)-4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl]-5-methoxymethylnicotinic acid (common name: imazamox) andb) at least one herbicide selected from the group consisting of 5-(2-chloro-α,α,α-trifluoro-p-tolyloxy)-N-mesyl-2-nitrobenzamide (common name: fomesafen), 2′,4′-dichloro-5′-(4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl)methanesulfonanilide (common name: sulfentrazone) and 3-isopropyl-1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide (common name: bentazone).
Owner:BASF AGROCHEMICAL PROD BV
Cleaning agent for oil-based drilling fluid as well as preparation method and evaluation method thereof
ActiveCN106634913AAbundant resourcesThe evaluation method is simpleFlow propertiesMaterial flash-point2-ImidazolineCleansing Agents
The invention discloses a cleaning agent for an oil-based drilling fluid as well as a preparation method and evaluation method thereof. The cleaning agent is prepared from the following components in percentage by weight: 50-60% of tetraethylenepentamine, 20-25% of fatty acid alkanol amide, 10-15% of 2-methyl-2-imidazoline and 5-10% of dimethylacetamide. The preparation method of the cleaning agent comprises the steps of: adding the tetraethylenepentamine into a reactor according to the percentage by weight, starting stirring, dripping fatty acid alkanol amide and dimethylacetamide, then adding 2-methyl-2-imidazoline, after continuing stirring for 5min, heating the mixture to 45-50 DEG C, stirring for 30-60min at constant temperature, and cooling the mixture to room temperature to obtain the cleaning agent. The evaluation method of the cleaning agent comprises determination of drainage time: (1) preparation of a standard test fluid; (2) preparation of a pulp sample; and (3) determination of the drainage time. The cleaning agent prepared by the preparation method is excellent in temperature resistance, and has an excellent cleaning effect for the residual oil-based drilling fluid which has complex components, a stable rubber matrix structure and high adhesion strength.
Owner:广汉市福客科技有限公司
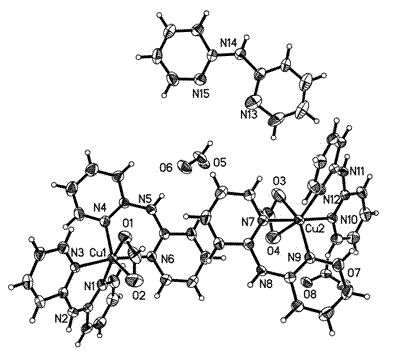
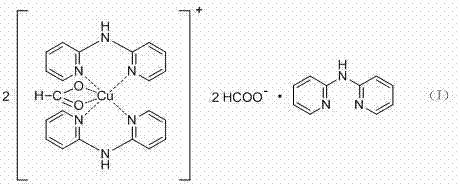
2, 2'-bi-pyridine amine copper complex and application thereof in 2-imidazoline derivative synthesis
InactiveCN103204863ASimple preparation processLower synthesis costOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compounds2-ImidazolineReusability
The invention discloses a complex shown by a structural formula (I). The complex is obtained by adding formic acid into soluble copper salt and 2, 2'-pyridine amine to adjust pH value to 5, heating and refluxing. The complex can be taken as a catalyst to be used for synthesis of 2-imidazoline and derivatives thereof, and has the advantages of mild reaction condition, high yield, short reaction time, simplicity and convenience in post-treatment, low catalyst price, reusability, freeness of solvent interference, and the like.
Owner:NORTHWEST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com