2, 2'-bi-pyridine amine copper complex and application thereof in 2-imidazoline derivative synthesis
A technology of copper complexes and complexes, applied in the direction of copper organic compounds, organic compounds/hydrides/coordination complex catalysts, chemical/physical processes, etc., can solve serious environmental pollution, low yield, long reaction time, etc. problems, to achieve the effect of simple preparation process, less dosage, and little influence on separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
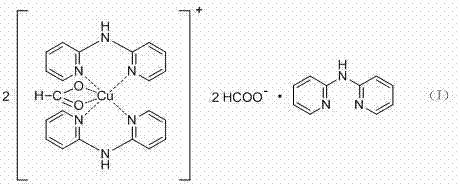
[0020] Embodiment 1: Synthesis of complexes shown in structural formula (I)
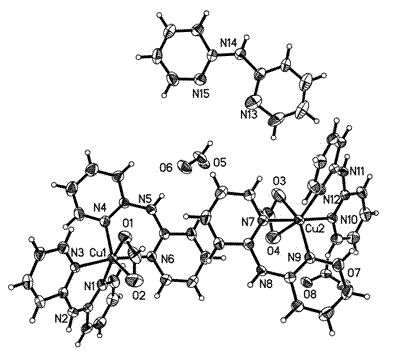
[0021] Weigh 0.20g of copper acetate and 0.17g of 2,2'-dipyridylamine, dissolve in 100mL of methanol:water (V / V)=1:1 solution, add formic acid dropwise to adjust the pH value to 5, heat to reflux for 2h, Filter the clear liquid in a Erlenmeyer flask, cool and crystallize to obtain the product. The test data of the single crystal structure is: Molecular formula C 54 h 49 Cu 2 N 15 o 8 , which belongs to the monoclinic crystal system, Cc space group, and the unit cell parameters are a = 11.5409(7)?, b = 15.3626(9)?, c = 30.2072(18)?, α = 90 (deg), β =90.4940(10) deg, gamma = 90 deg.
Embodiment 2
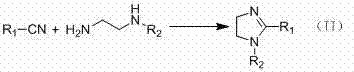
[0022] Embodiment 2: Synthesize 2-phenylimidazoline with benzonitrile and ethylenediamine as raw materials.
[0023] Add 5mmol of benzonitrile, 20mmol of ethylenediamine and 1mmol of the catalyst prepared in Example 1 into a 50mL round bottom flask, and heat to reflux for 6h under stirring. After the reaction is complete, add 20 mL of CH 2 Cl 2 , after filtering off the catalyst, the CH 2 Cl 2 Evaporate to dryness to obtain 2-phenylimidazoline crude product, column separation (eluent: ethyl acetate / methanol (V / V)=3:1) obtains 2-phenylimidazoline pure product 0.63g, yield is 86 %. Mass Spectrum (m / z): 147 [M+H] + . NMR: 1 H NMR (400MHz, CDCl 3 ): δ 3.78 (s, 4H, 2CH 2 ), 4.78 (br s, 1H, NH), 7.39-7.45 (m, 3H, ArH), 7.76-7.80 (m, 2H, ArH). Infrared (KBr): ν / cm -1 : 3201, 2929, 2867, 1611, 1571, 1508, 1469, 1344, 1269, 981, 778, 696.
Embodiment 3
[0024] Embodiment 3: take p-chlorobenzonitrile and ethylenediamine as raw material synthesis 2-(p-chlorophenyl) imidazoline
[0025] Add 5mmol of p-chlorobenzonitrile, 20mmol of ethylenediamine and 1mmol of the catalyst prepared in Example 1 into a 50mL round bottom flask, and heat to reflux for 6h under stirring. After the reaction is complete, add 20 mL of CH 2 Cl 2 , after filtering off the catalyst, the CH 2 Cl 2 Evaporate to dryness to obtain 2-(p-chlorophenyl) imidazoline crude product, column separation (eluent: ethyl acetate / methanol (V / V)=3:1) obtains 2-(p-chlorophenyl) imidazoline pure Product 0.70g, the yield is 78%. Mass Spectrum (m / z): 181 [M+H] + . NMR: 1 H NMR (CDCl 3, 400MHz) δ: 3.80 (s, 4H, 2CH 2 ), 7.38 (d, 2H, ArH), 7.73 (d, 2H, ArH). Infrared (KBr): ν / cm -1 : 3235, 2697, 2361, 1605, 1558, 1518, 1483, 1351, 1274, 1125, 1089, 986, 838, 727.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com