Method for synthesizing phenylacetic acid from benzyl chloride carbonyl
A benzyl chlorocarbonyl compound synthesis technology, applied in the field of benzyl chlorocarbonylation to synthesize phenylacetic acid, can solve the problems of large dosage, low recycling rate, environmental pollution, etc., and achieve low toxicity, enhanced water solubility, and excellent preparation process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
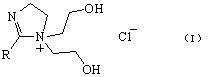
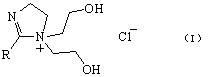
[0019] Embodiment 1: Synthesis and related characterization of phase transfer catalyst
[0020] Add 0.10 mol acetic acid and 0.12 mol N-(2-hydroxyethyl) ethylenediamine to a 100 ml three-necked flask, control the pressure at 26665 Pa, and gradually increase the reaction temperature to 200 °C, and about 1.8 ml (0.1 mol) of water Steam out. Then the system pressure was gradually reduced to 3333Pa, and about 1.8 ml (0.1mol) of water was distilled out during the reaction. Cooling under reduced pressure gave a pale yellow solid, and recrystallized to give a white solid, which was the imidazoline intermediate.
[0021] Add 10 mmol of imidazoline intermediate and 20 ml of DMF into a 100 ml three-necked flask, and heat up to 80°C. Under stirring, 11 mmol chloroethanol was slowly added dropwise. After the addition was completed, the reaction was continued for 5h. The solvent was evaporated, and 2-methyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride was obtained by column s...
Embodiment 2
[0025] 2-Methyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride as a phase transfer catalyst for synthesis of phenylacetic acid by carbonylation of benzyl chloride
[0026] Add 1 mmol 2-methyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride phase transfer catalyst in a 250 ml three-necked flask, and add 5 mmol Co(PPh 3 ) 2 Cl 2 As the main catalyst, 2.5 mmol of triphenylphosphine as the co-catalyst, and 50 ml of xylene as the solvent, the reaction was carried out at normal temperature and pressure for 10 minutes. Add 50 mmol benzyl chloride, 40ml 30% NaOH solution, and react at 60°C for 24h. The organic phase was removed, and the aqueous phase was adjusted to pH=2 with 10% hydrochloric acid solution. The resulting solution is suction filtered to obtain phenylacetic acid in the form of white flaky crystals. Yield reaches 78.5%. 99% purity. NMR, mass spectrometry, infrared and elemental analysis data were consistent with the report.
Embodiment 3
[0028] 2-Methyl-1-bis(2-hydroxyethyl)-2-imidazoline chloride as a phase transfer catalyst for synthesis of phenylacetic acid by carbonylation of benzyl chloride
[0029] Similar to Example 2, the difference is that: the used phase transfer catalyst solution and 40ml of 30% NaOH solution recovered in the example were added to the prepared cobalt carbonyl catalyst, and the rest of the reaction conditions were the same. The yield of phenylacetic acid was 73.3%. 99% purity. NMR, mass spectrometry, infrared and elemental analysis data were consistent with the report.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com