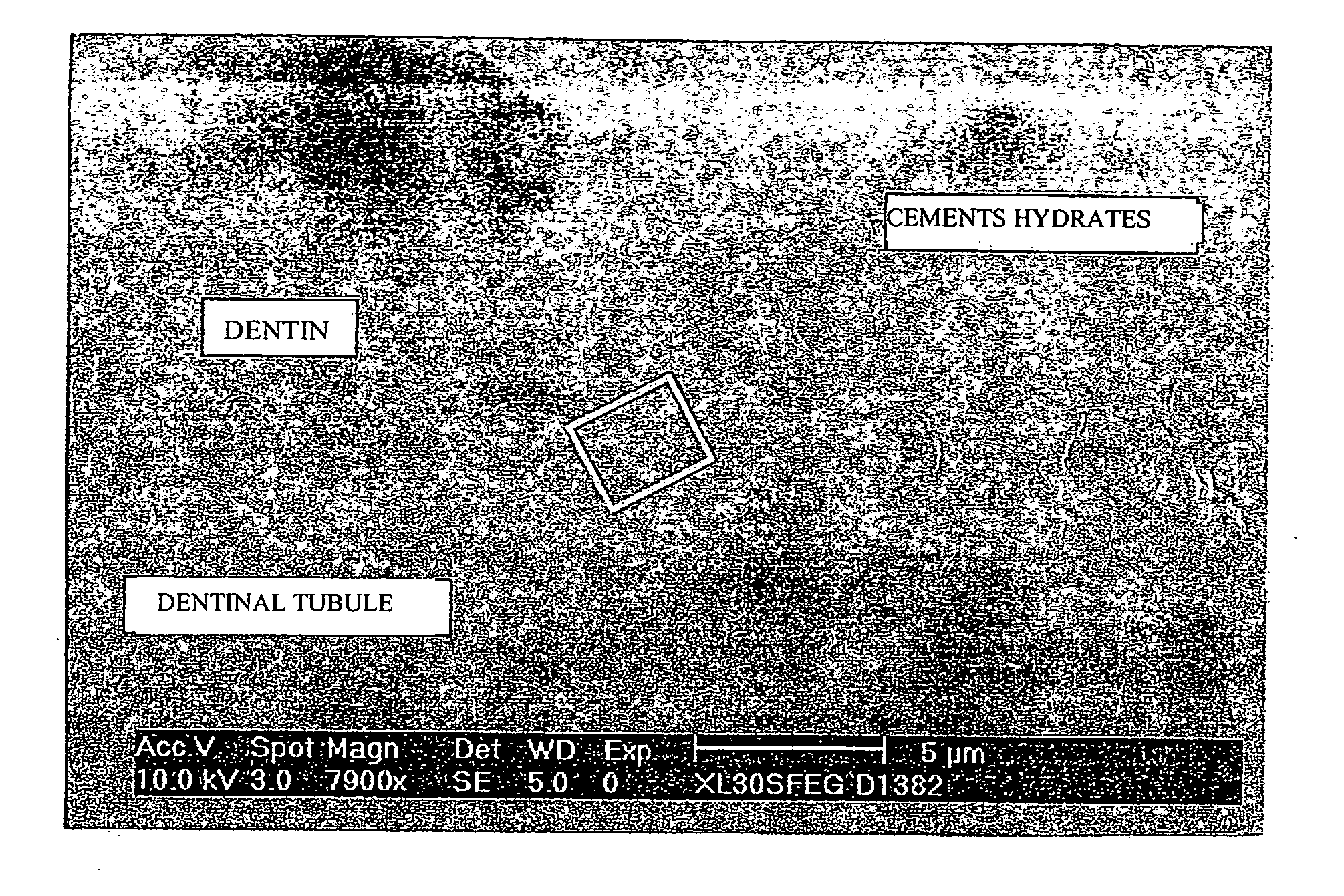
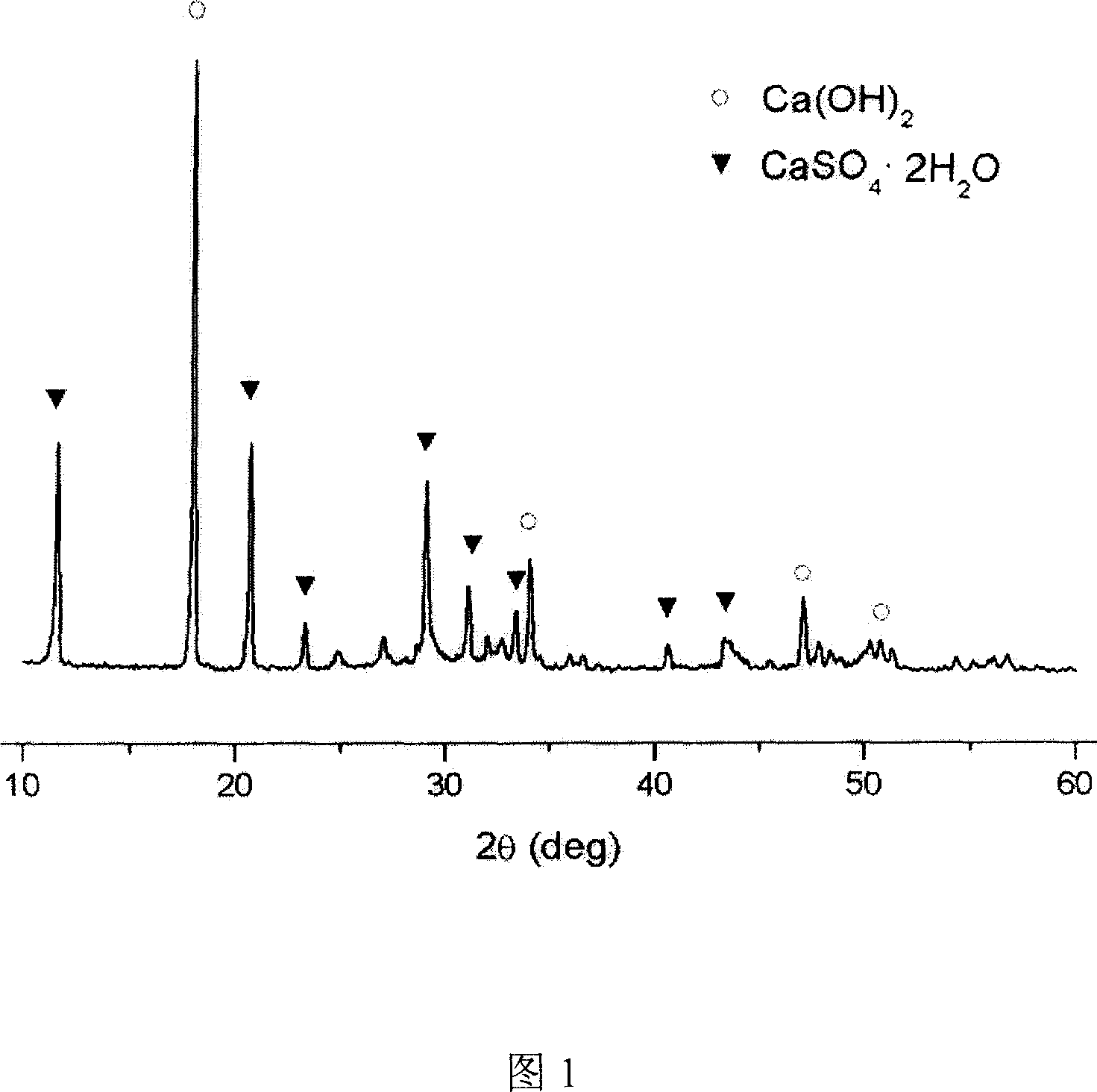
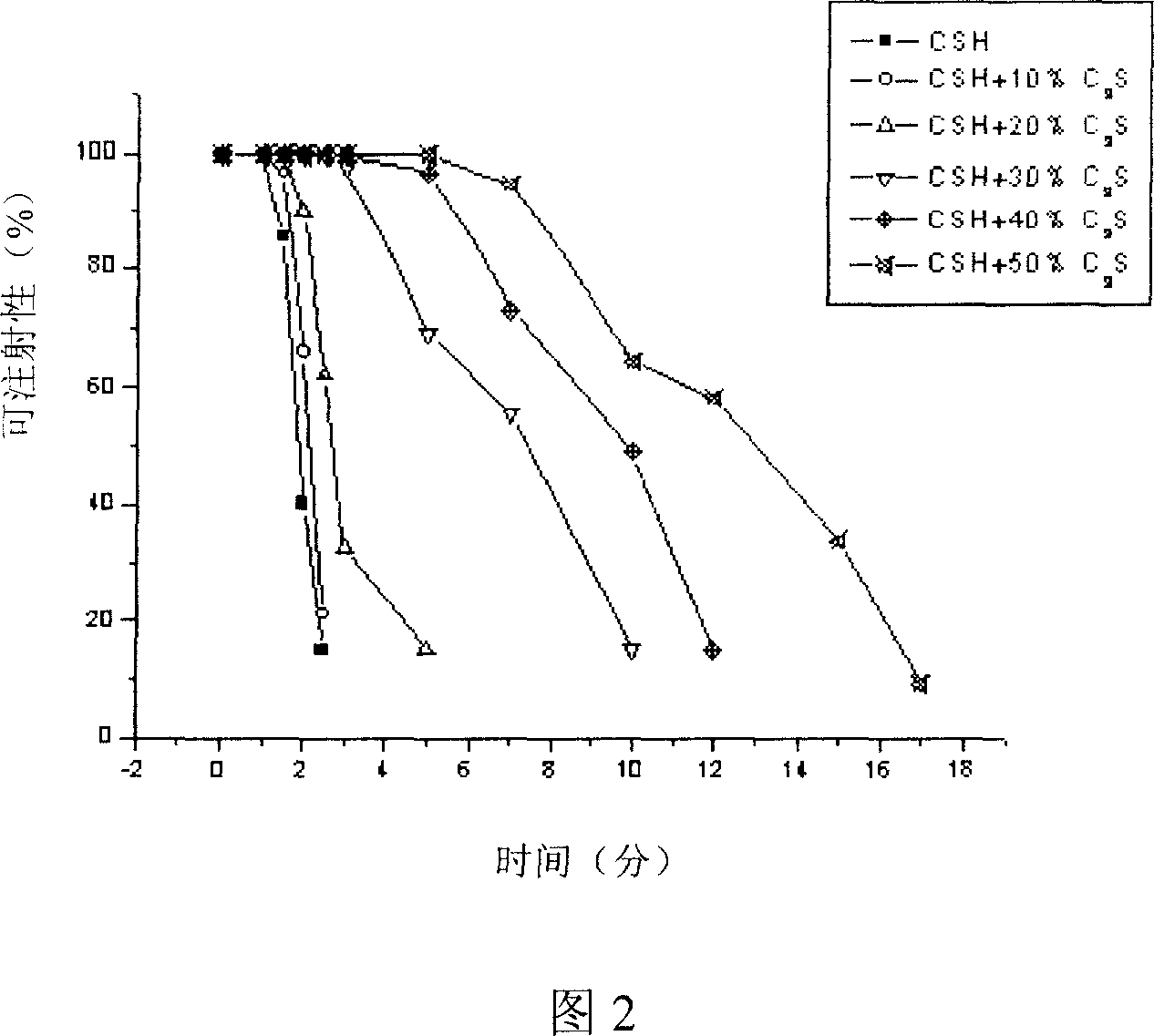
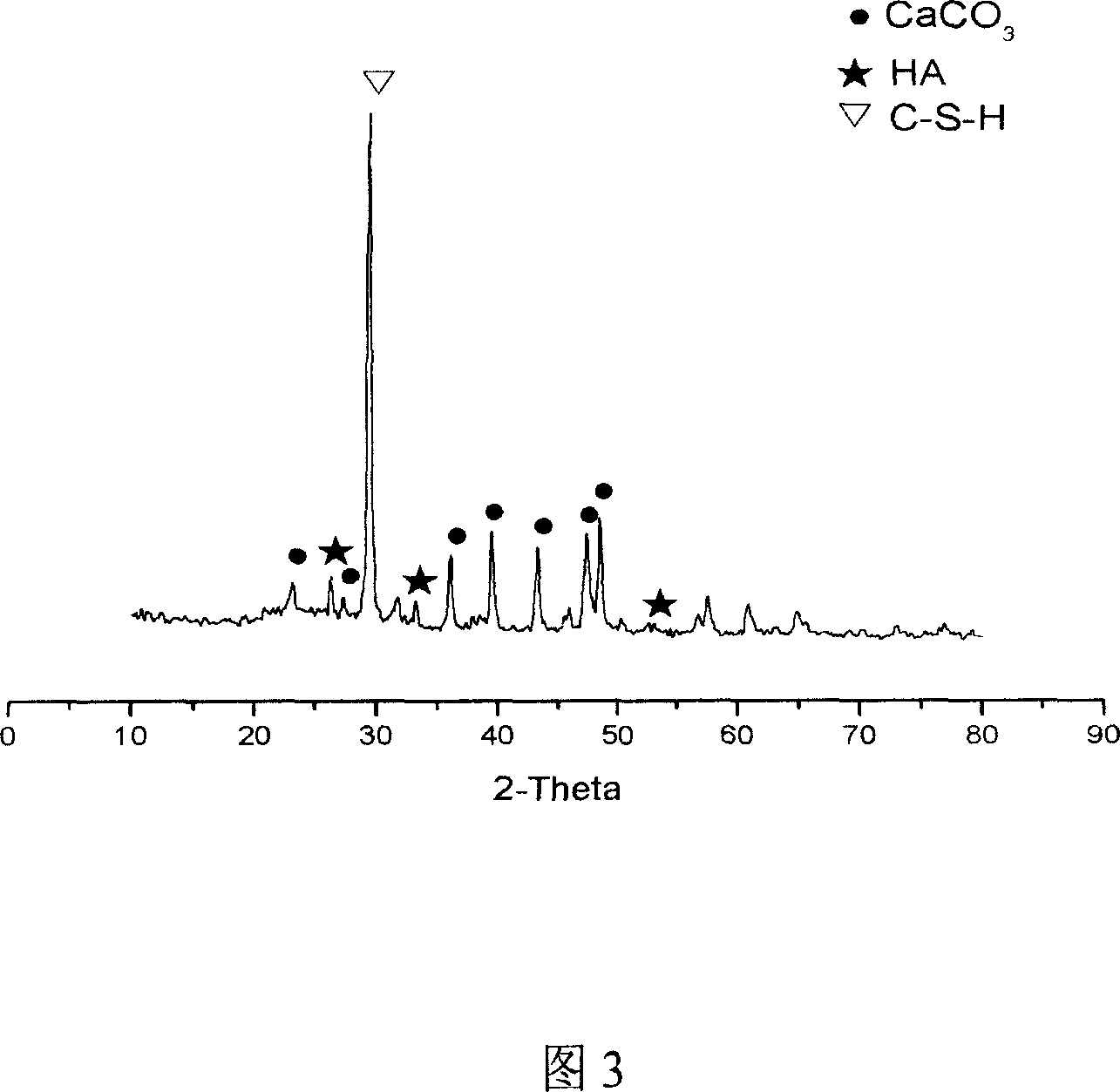
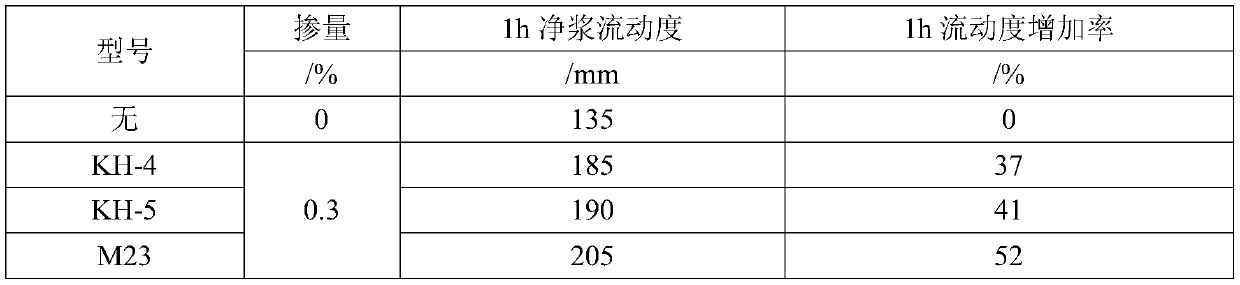
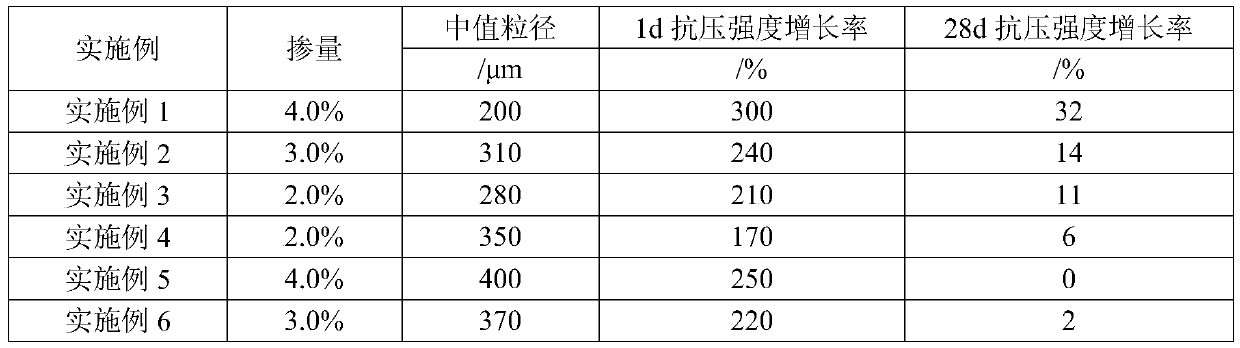
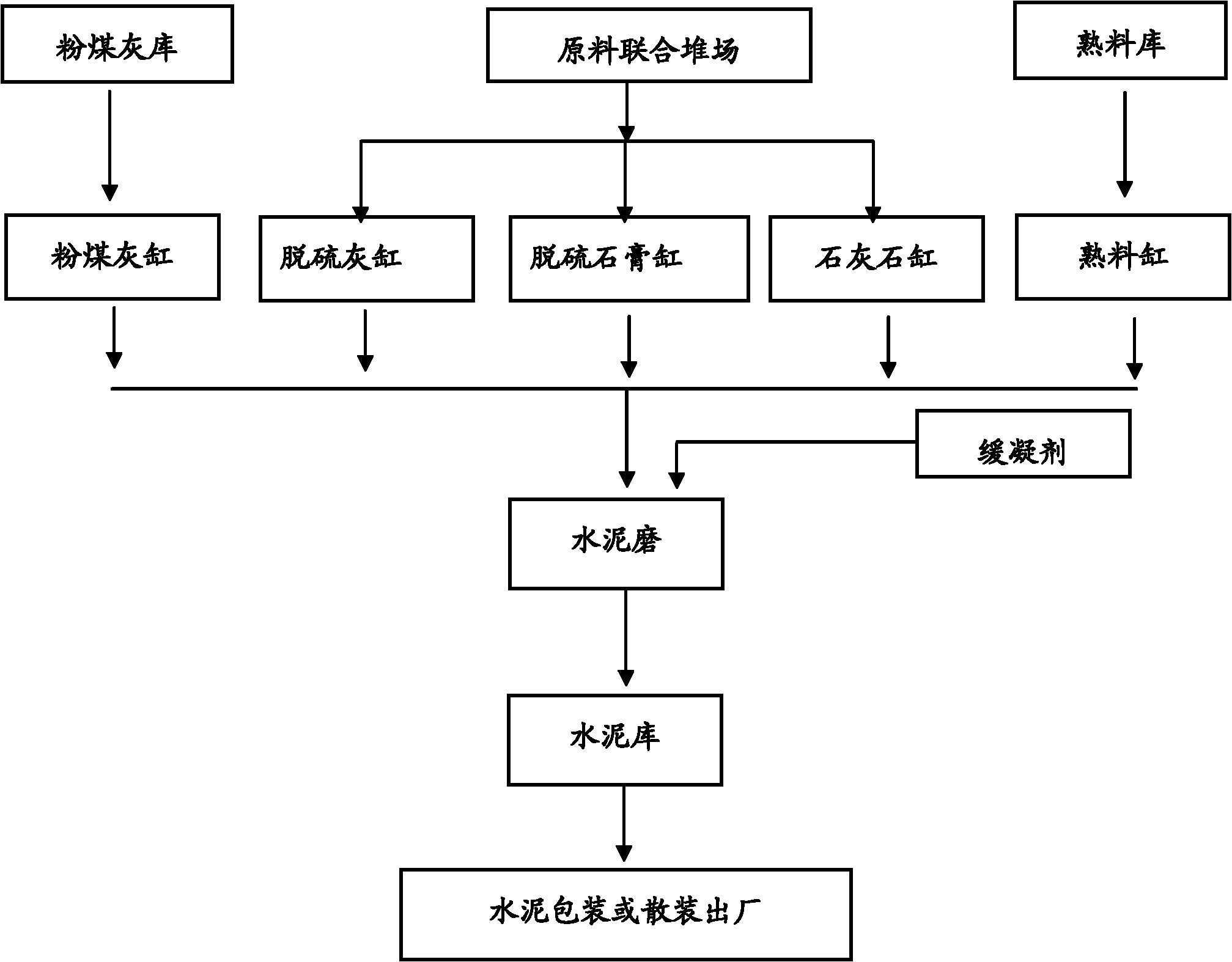
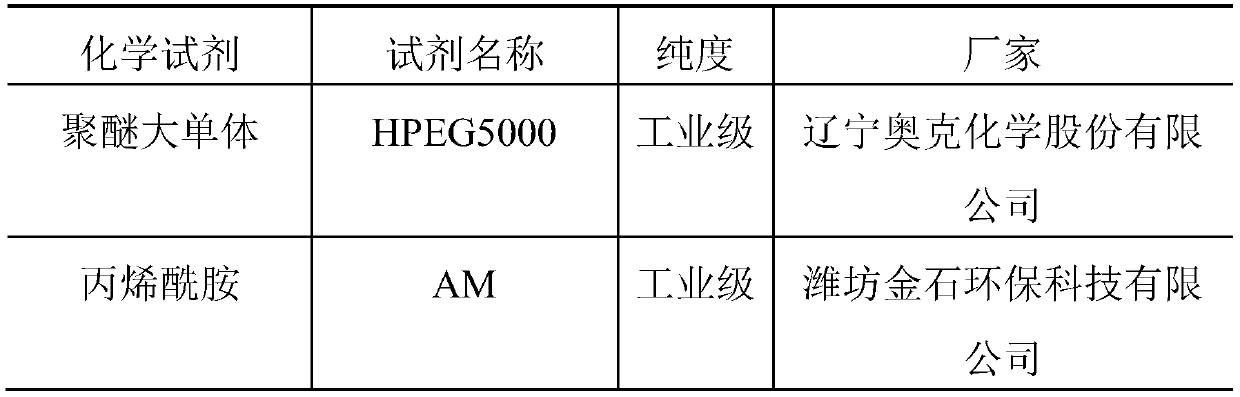
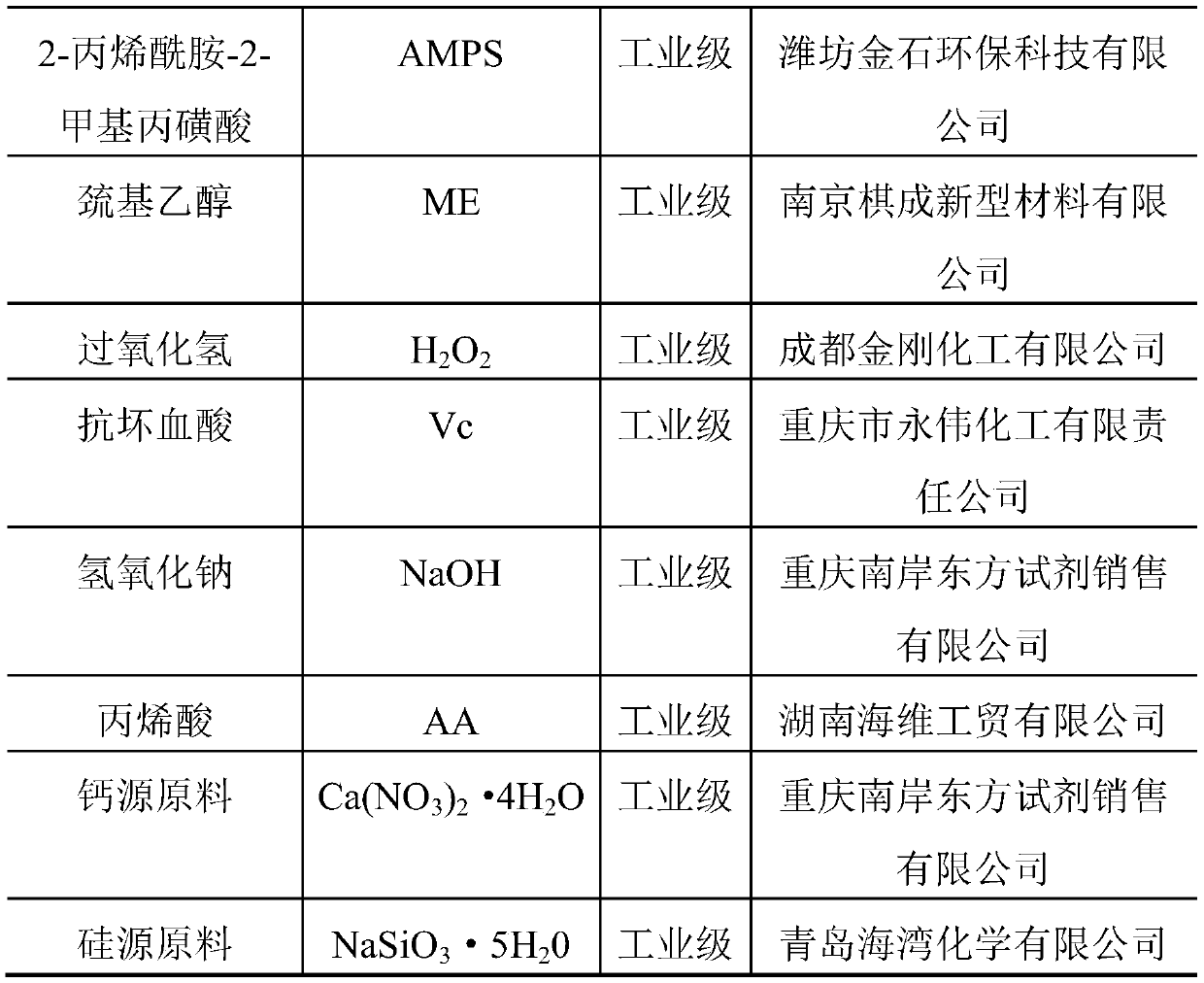
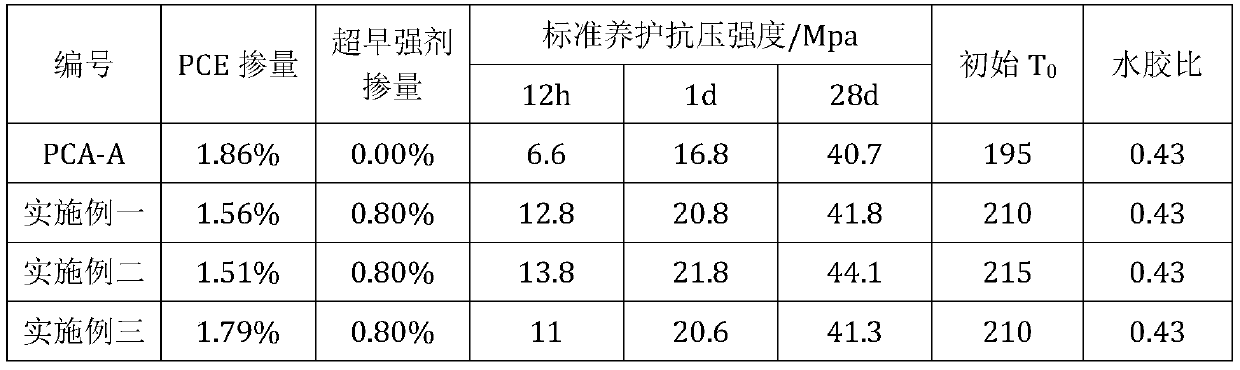
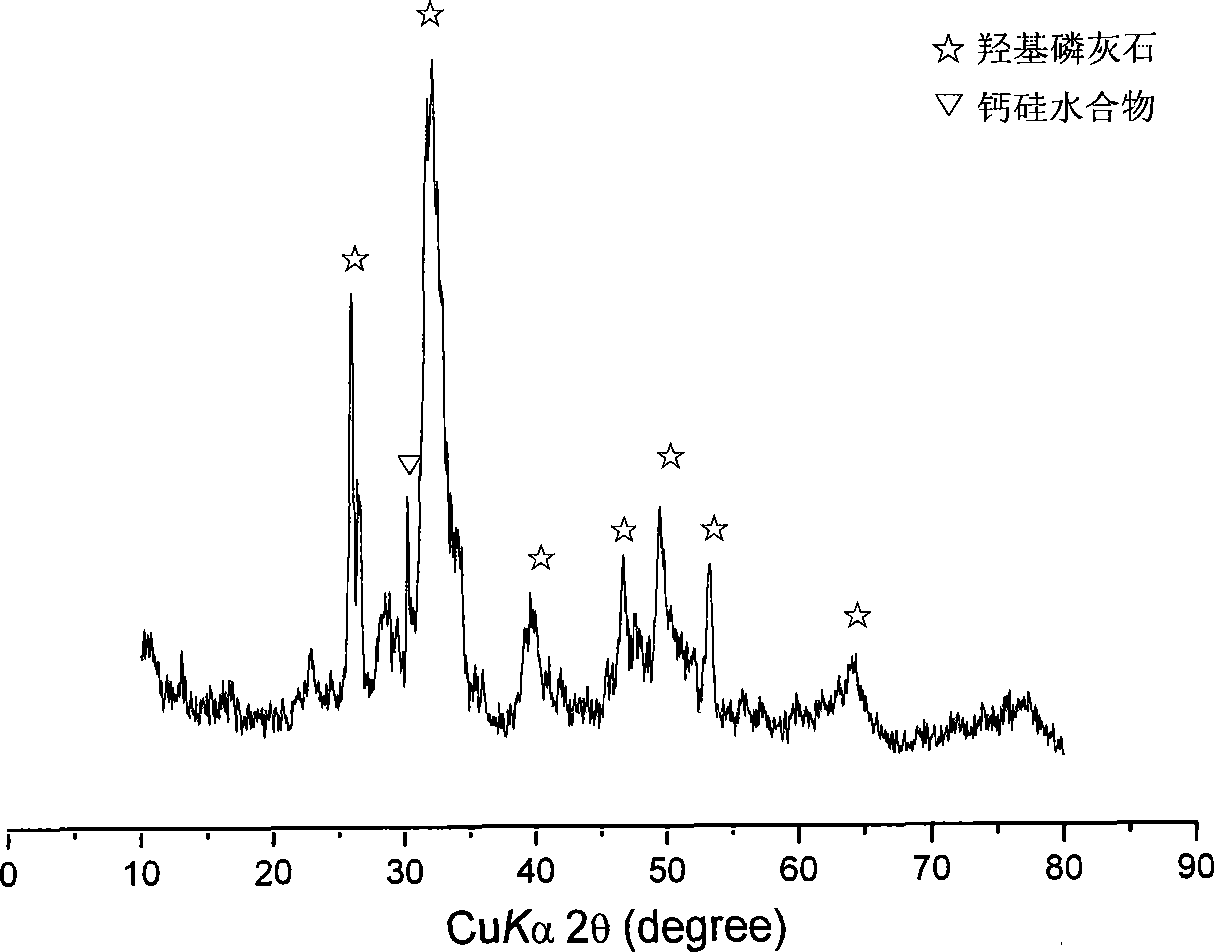
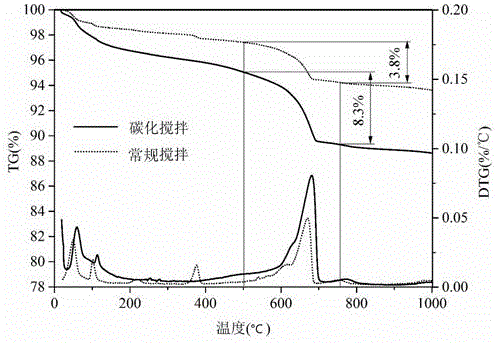
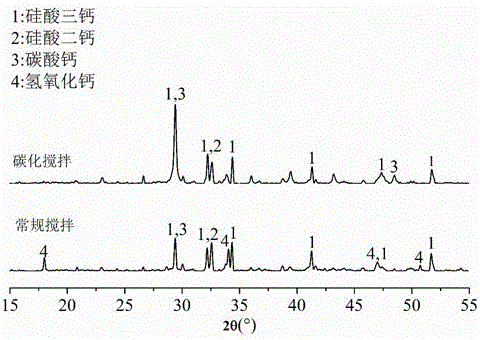
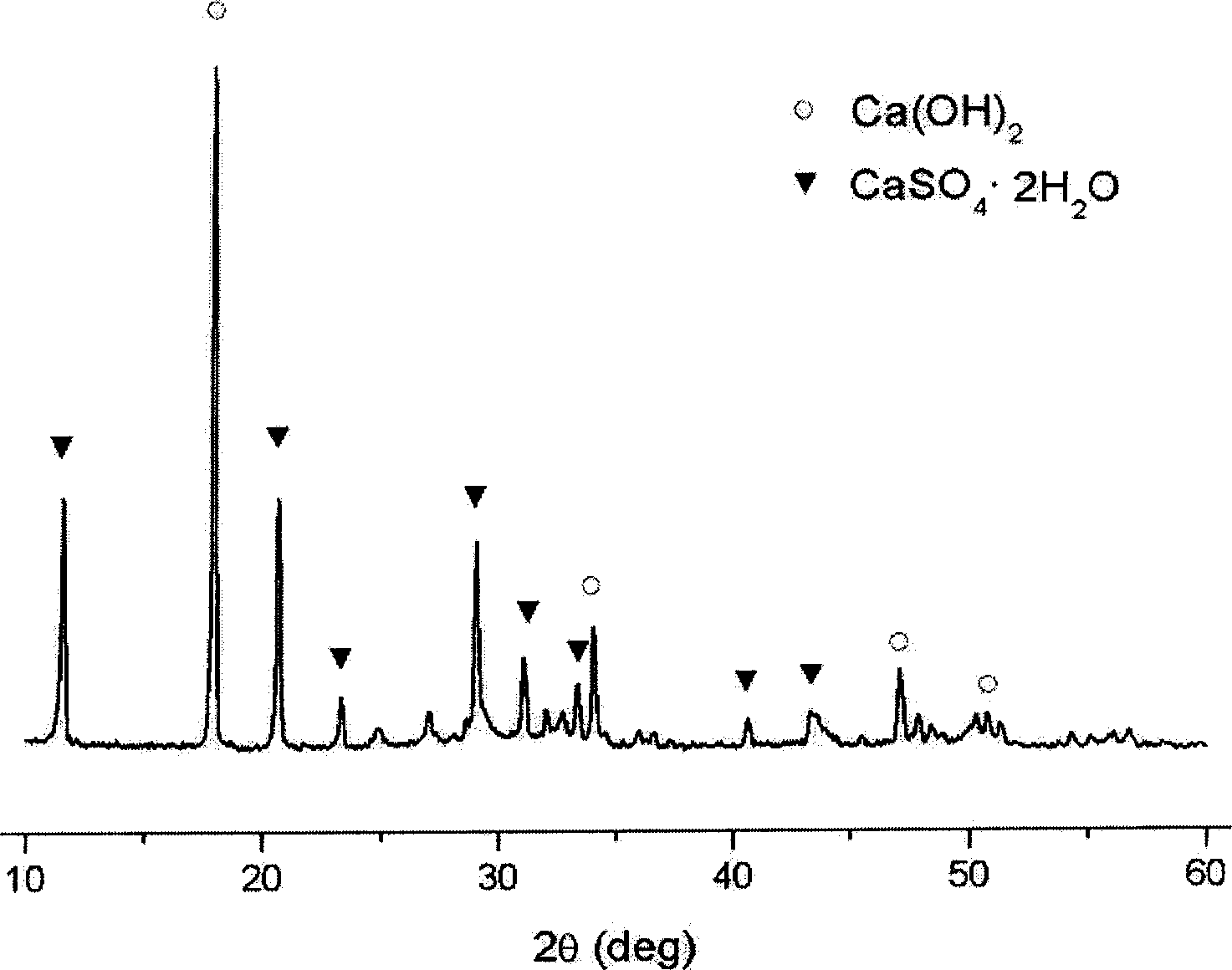
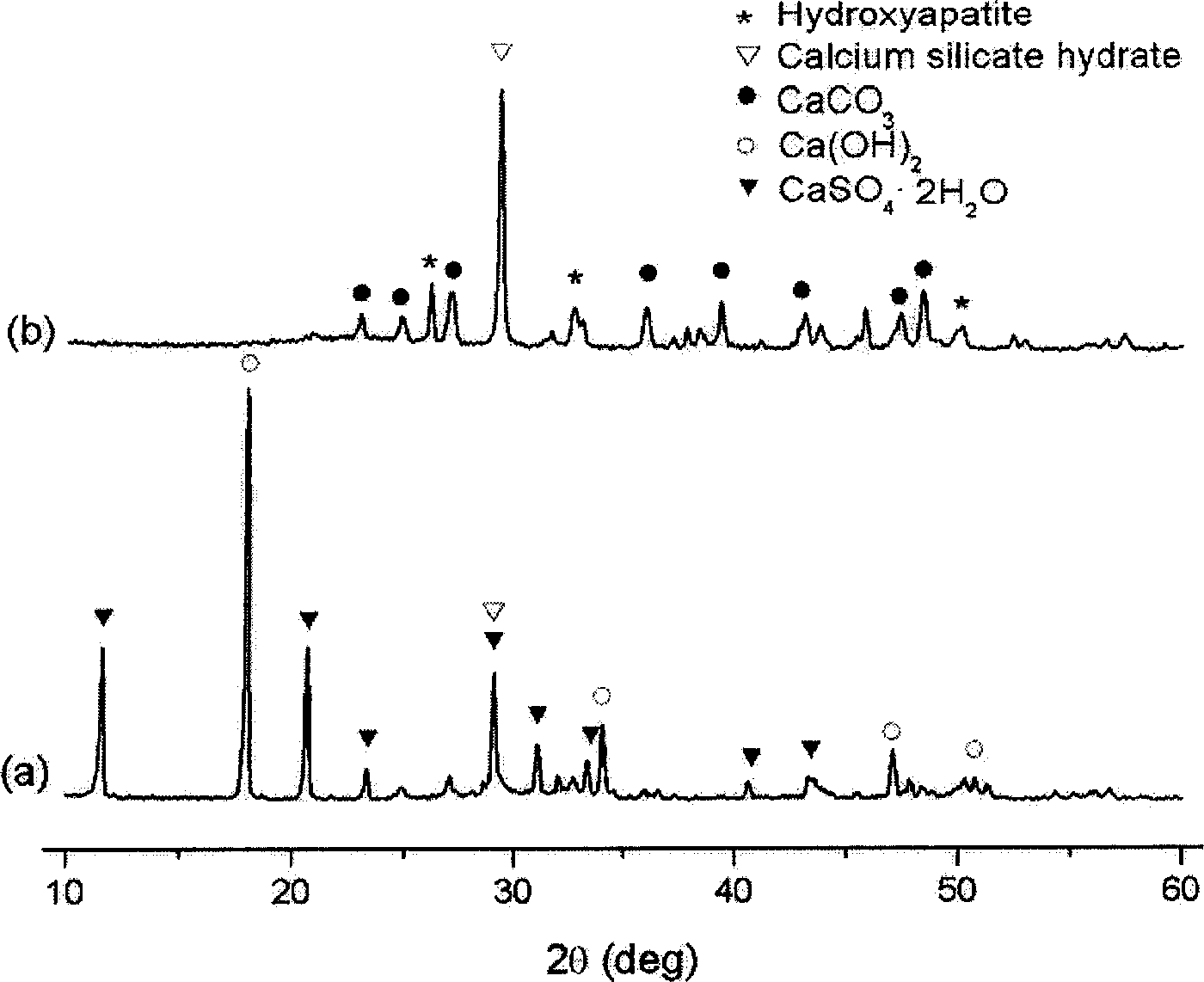
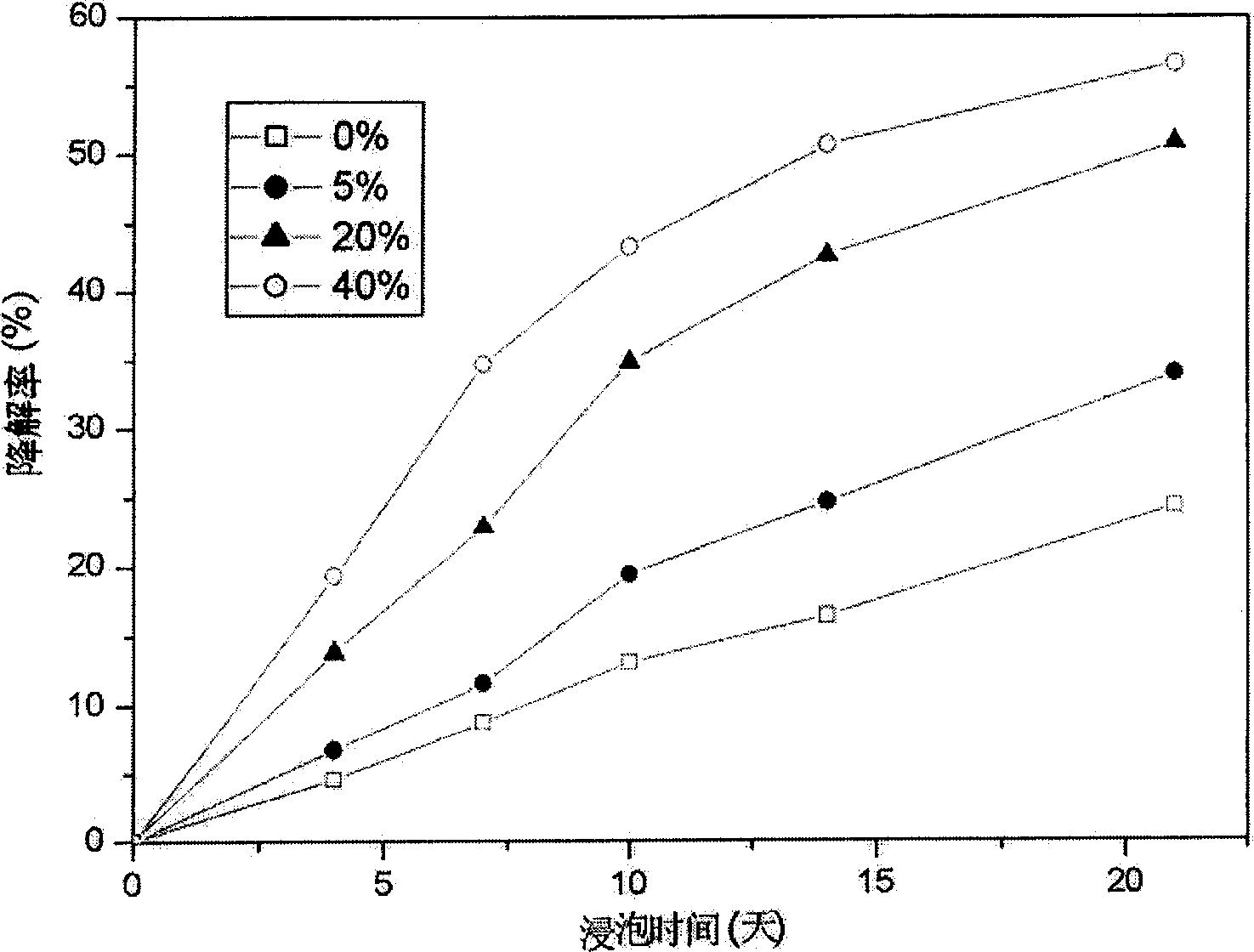
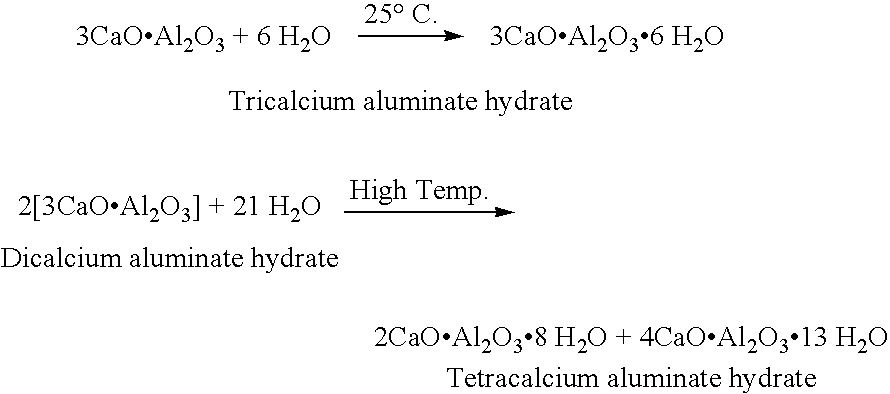
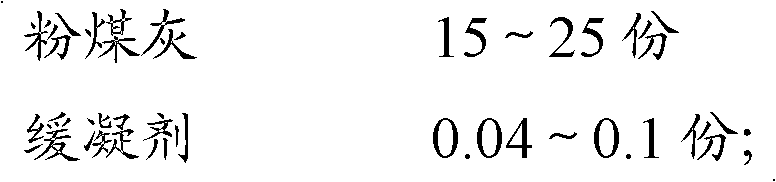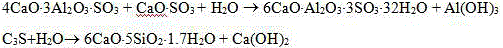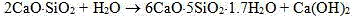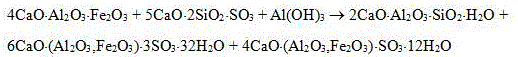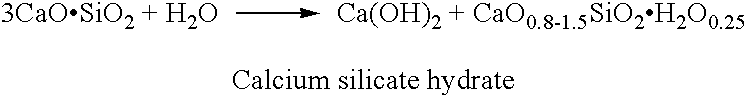Patents
Literature
255 results about "Tricalcium silicate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
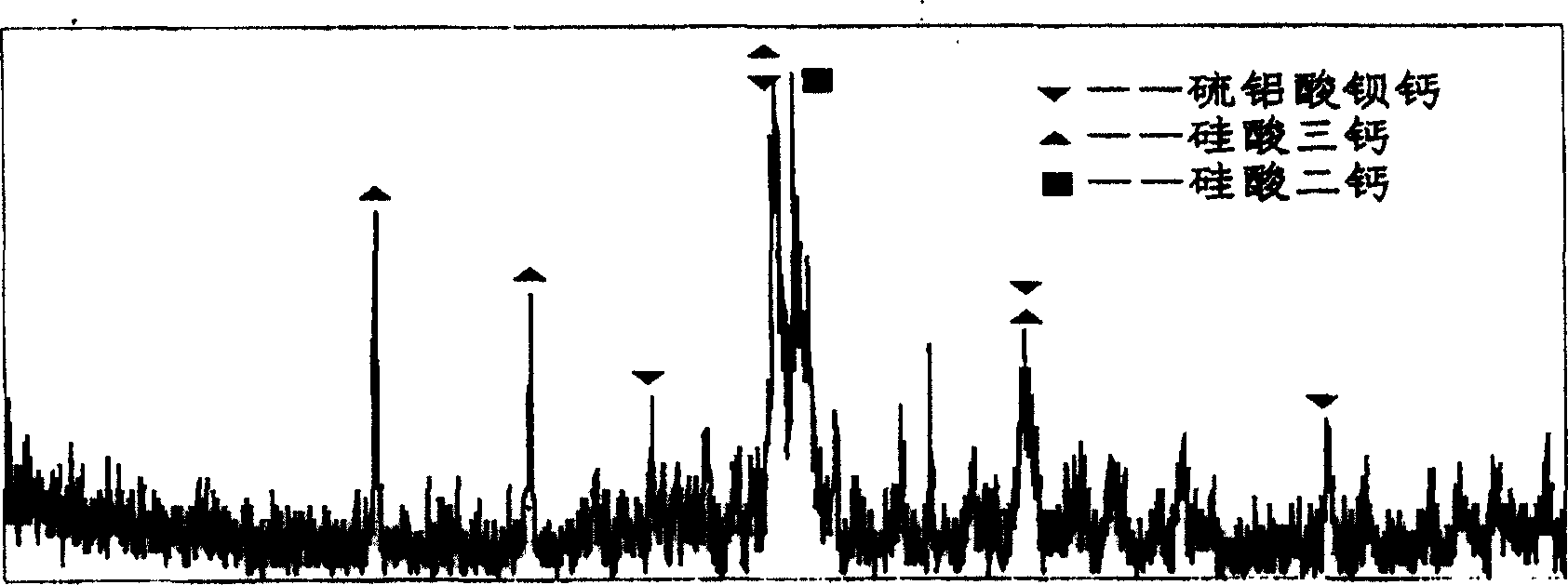
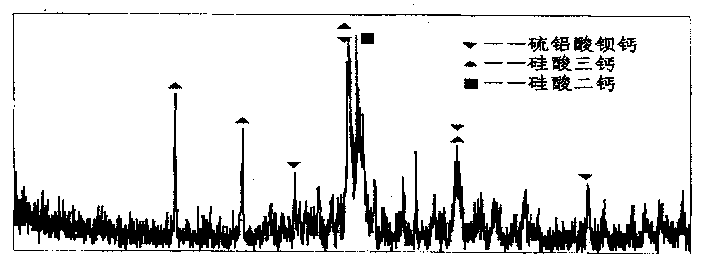
Alite is an impure form of tricalcium silicate, Ca 3 SiO 5, sometimes formulated as 3CaO·SiO 2 (C 3 S in cement chemist notation, CCN) with typically 3-4% of substituent oxides. It is the major, and characteristic, mineral phase in Portland cement .
Method for preparing building material products through hydration-carbonation coupling technique
The invention belongs to the technical field of building materials, and provides a method for preparing building material products through processing industrial solid waste by hydration-carbonation coupling technique. The method comprises steps of uniformly mixing the industrial waste comprising at least one of calcium oxide, calcium hydroxide, dicalcium silicate, tricalcium silicate and tobermorite with alkaline excitation material and proper amount of water so as to prepare the blank of the building material product, wherein the industrial waste comprises steel slag, mineral waste residue, furnace slag, coal ash or coal gangue, the alkaline excitation material comprises carbide slag, lime, Portland cement or waste cement; maintaining for a period through hydration, then maintaining through carbonation so as to obtain the carbonate-based building material product. The coupling technique can effectively use the industrial solid wastes such as steel slag, mineral waste residue, furnace slag, coal ash, coal gangue, carbide slag and the like, so that emission of greenhouse gases is reduced, the greenhouse effect is relieved, furthermore, the method can be used for producing the building material products with good properties, effectively uses the waste and is environmentally friendly.
Owner:DALIAN UNIV OF TECH
Self-setting composite bone repair material for human body hard tissue repair and application
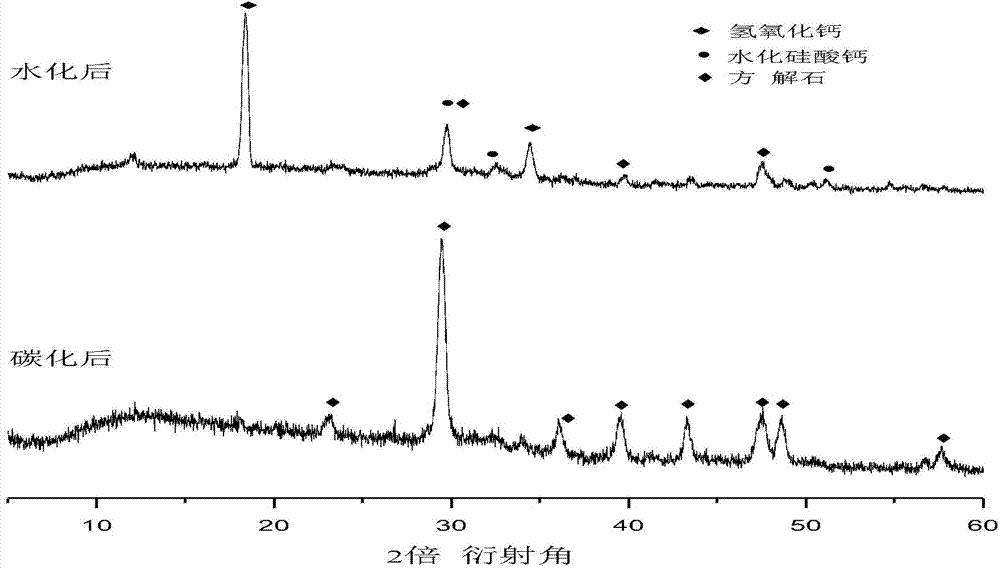
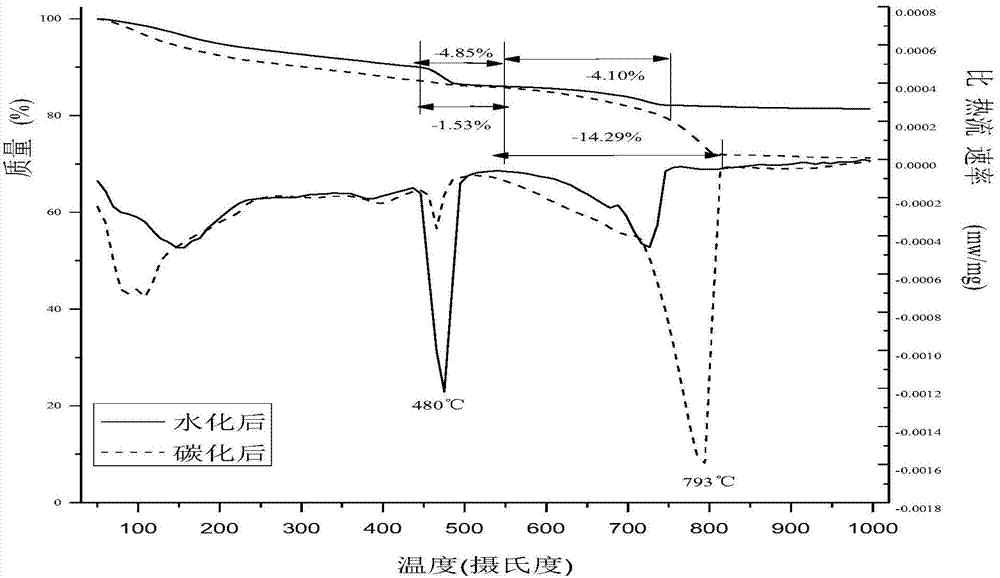
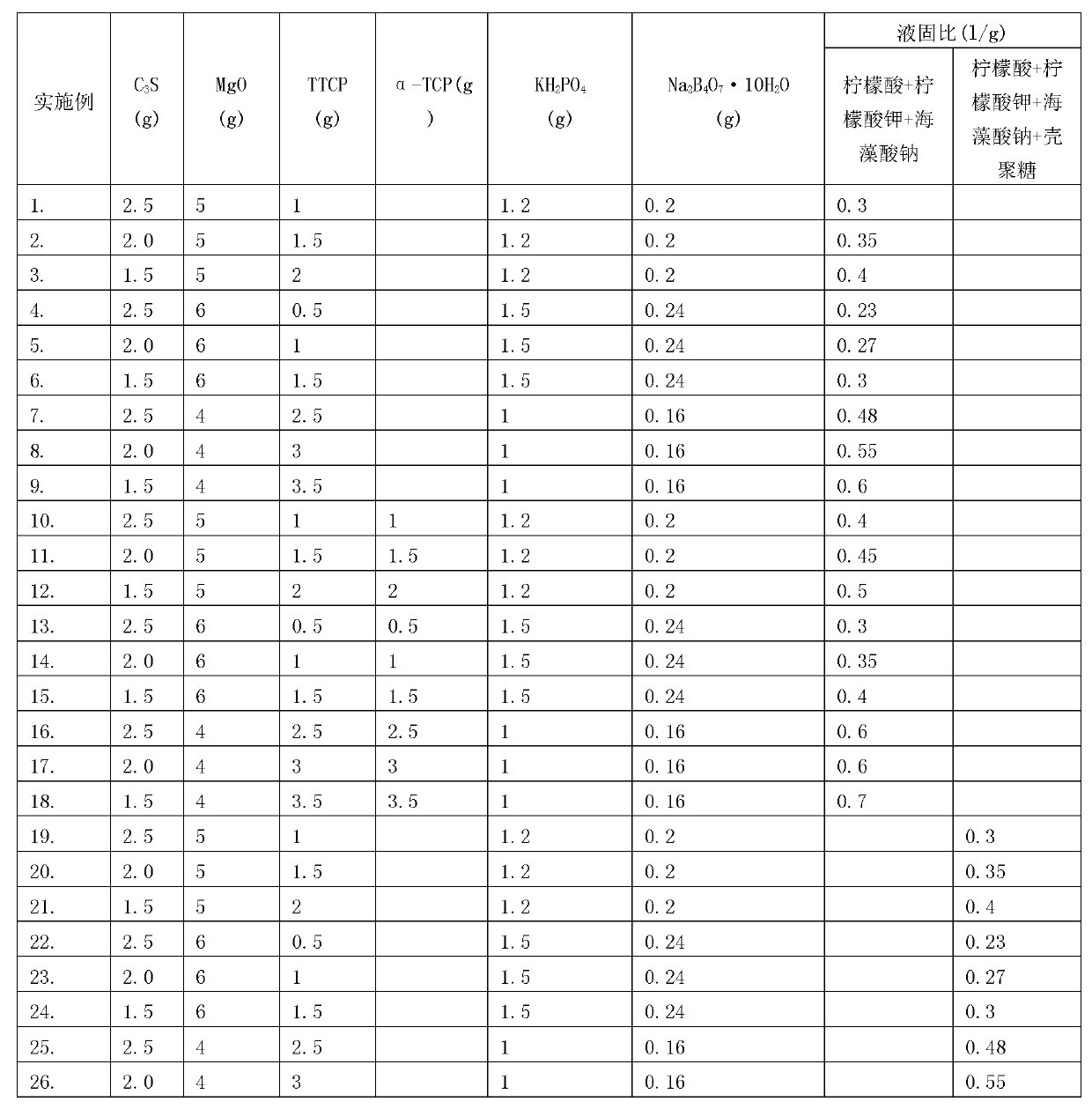
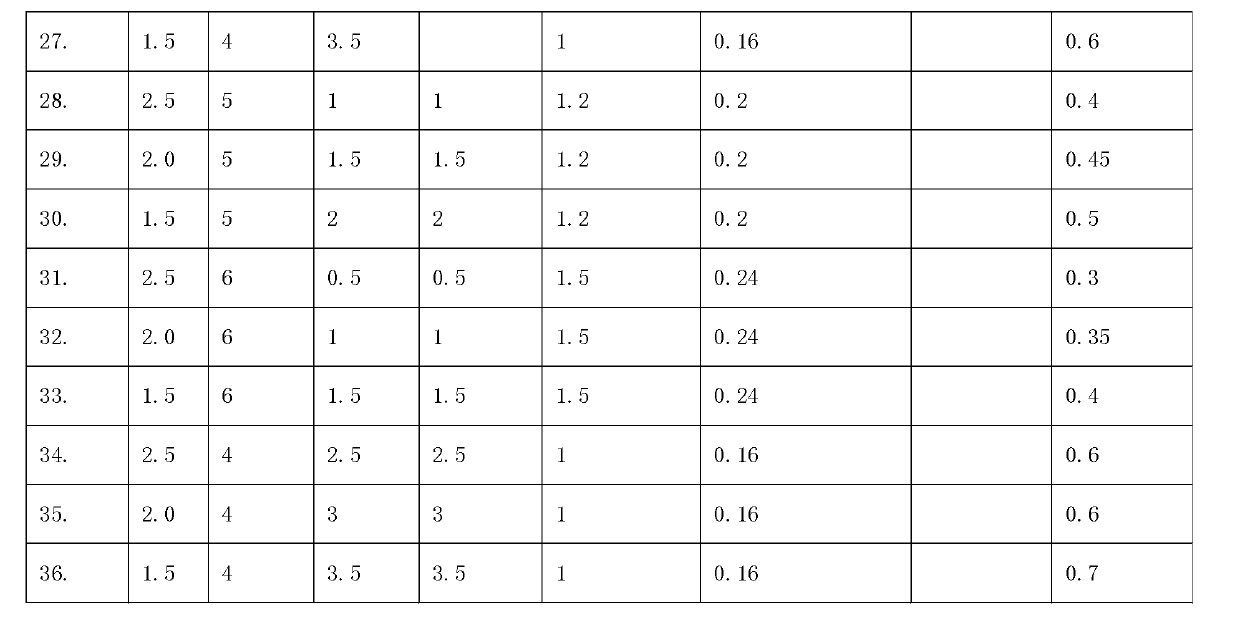
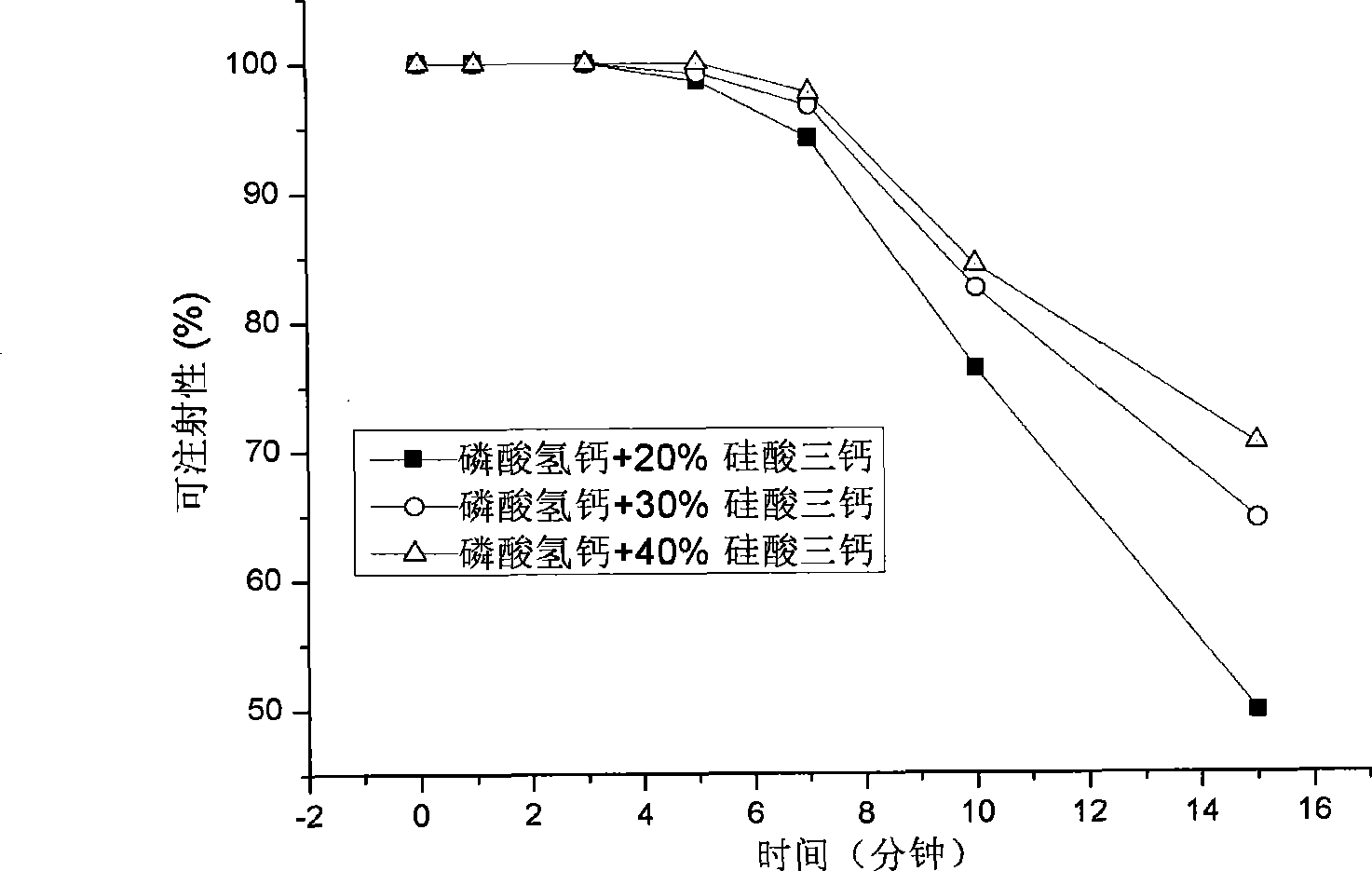
The invention discloses a self-setting composite bone repair material for human body hard tissue repair and application. The self-setting composite bone repair material comprises solid-phase powder and a liquid phase, wherein the solid-phase powder contains 5-20% of tricalcium silicate, 30-70% of electric smelting magnesium oxide, 5-30% of calcium phosphate bone cement, 5-30% of potassium dihydrogen phosphate or ammonium dihydrogen phosphate, and retarder accounting for 2-10% of the weight of basic oxide, i.e. magnesium oxide; the liquid phase is selected from one or a mixture of more than one of deionized water, soluble phosphate, sodium alginate, citric acid, potassium citrate and chitosan; and the ratio of the liquid phase to the solid phase is 0.3-0.8ml / g, and the solid-phase powder and the liquid phase are uniformly blended. The bone cement material has good combination property; and as the initial setting time of the bone cement material is 3-20 minutes, the final settling time is 7-50 minutes, and the compressive strength is 10-50Mpa in 24 hours and 20-90Mpa in 72 hours, the bone cement material can meet the requirement of clinical application and is suitable for treating bone defect repair and osteoporosis and fixing and treating bone fractures in orthopedic departments, spine surgery, plastic surgery and stomatological departments.
Owner:HENAN POLYTECHNIC UNIV
Premixed biological hydraulic cement paste composition and using the same
ActiveUS20080299093A1Promote absorptionEnhanced X-ray imagingBiocideImpression capsCalcium silicateFiber
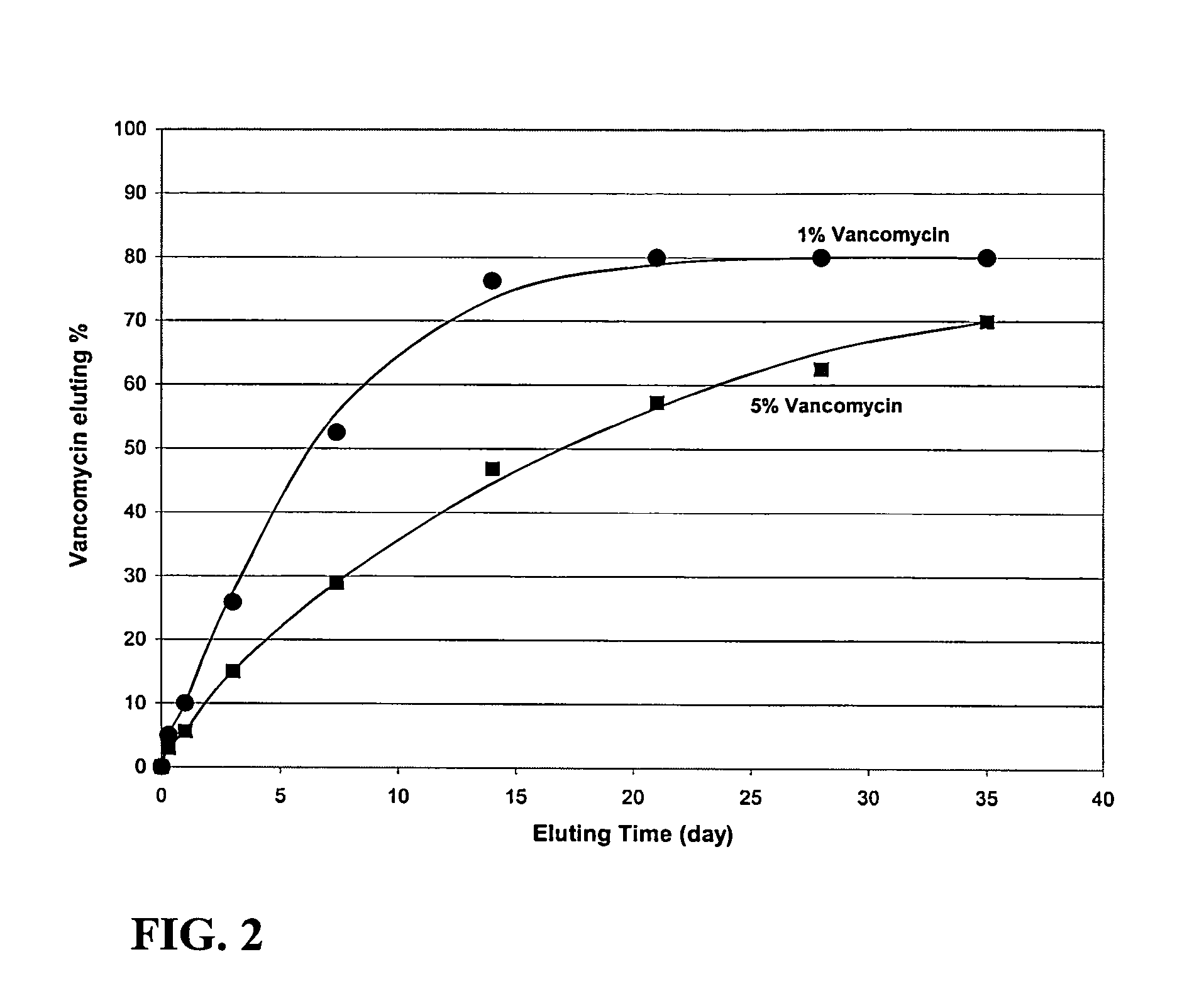
A premixed cement paste for use in medical or dental applications. The premixed cement paste remains fluid when stored in a hermetically sealed condition, but hydrates and hardens to set when placed in a physiological environment. The cement paste includes at least one calcium silicate compound and at least one substantially water-free liquid carrier mixed with the at least one calcium silicate compound; the substantially water-free liquid carrier avoids hydration of the mixture during storage, but undergoes exchange with aqueous physiological solutions so that the cement past hydrates and hardens to set when placed in a physiological environment. The paste may be placed in the physiological environment by injection, for example. The at least one calcium silicate compound may be, for example, calcium silicate, dicalcium silicate, tricalcium silicate, or mixtures thereof. The substantially water-free liquid may be, for example, ethylene glycol, polyethylene glycol, liquid glycerol, glycerin, ethyl alcohol, vegetable oil, animal oil, silicon oil, hydroxypropyl methylcellulose, or mixtures thereof. The substantially water-free liquid carrier preferably includes water in an amount less than about 20% by weight percent of the paste. The paste may include a secondary phase for enhanced properties, such as, for example, a fibrous or particulate material for enhanced mechanical properties, biodegradable or soluble materials that provide room for bone in-growth, bioactive materials such as antibiotics that elute into the physiological environment from the set cement, and radio-opaque materials that enhance X-ray imaging of the cement.
Owner:INNOVATIVE BIOCERAMIX
Deep water low temperature cementing cement system
InactiveCN101054513AImprove performanceRapid development of strengthDrilling compositionWell cementingCement slurry
The invention belongs to sea deep water complex well cementing which is suitable for low temperature, tending to superficial water-gassed-out. The mineral components and mass percent composition are: 23-45 wt.% of tricalcium silicate, 12-25 wt.% of calcium sulphoaluminate, 15-23 wt.% of dicalcium silicate, 3-10 wt.% of gypsum, 4-8 wt.% of calcium carbonate, and residual celite, calcium aluminate and other microelement. The using process of the deep water complex well cementing includes: adding 0-3 wt.% of coagulant, 0-1.2 wt.% of retarder, 15-45 wt.% of hollow microglobin and water to prepare a low density cement slurry system of 1.35-1.60g / cm3 which has a controllable gelled time and has a perfect right-angle gelling capacity, a set cement volume microdilatancy and develops fast at low temperature. The inventive deep water low temperature complex well cementing has a high early strength, a short curing time, a powerful channeling-preventing ability, and microdilatancy, which provides a powerful guarantee to improve the deep water complex well quality.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
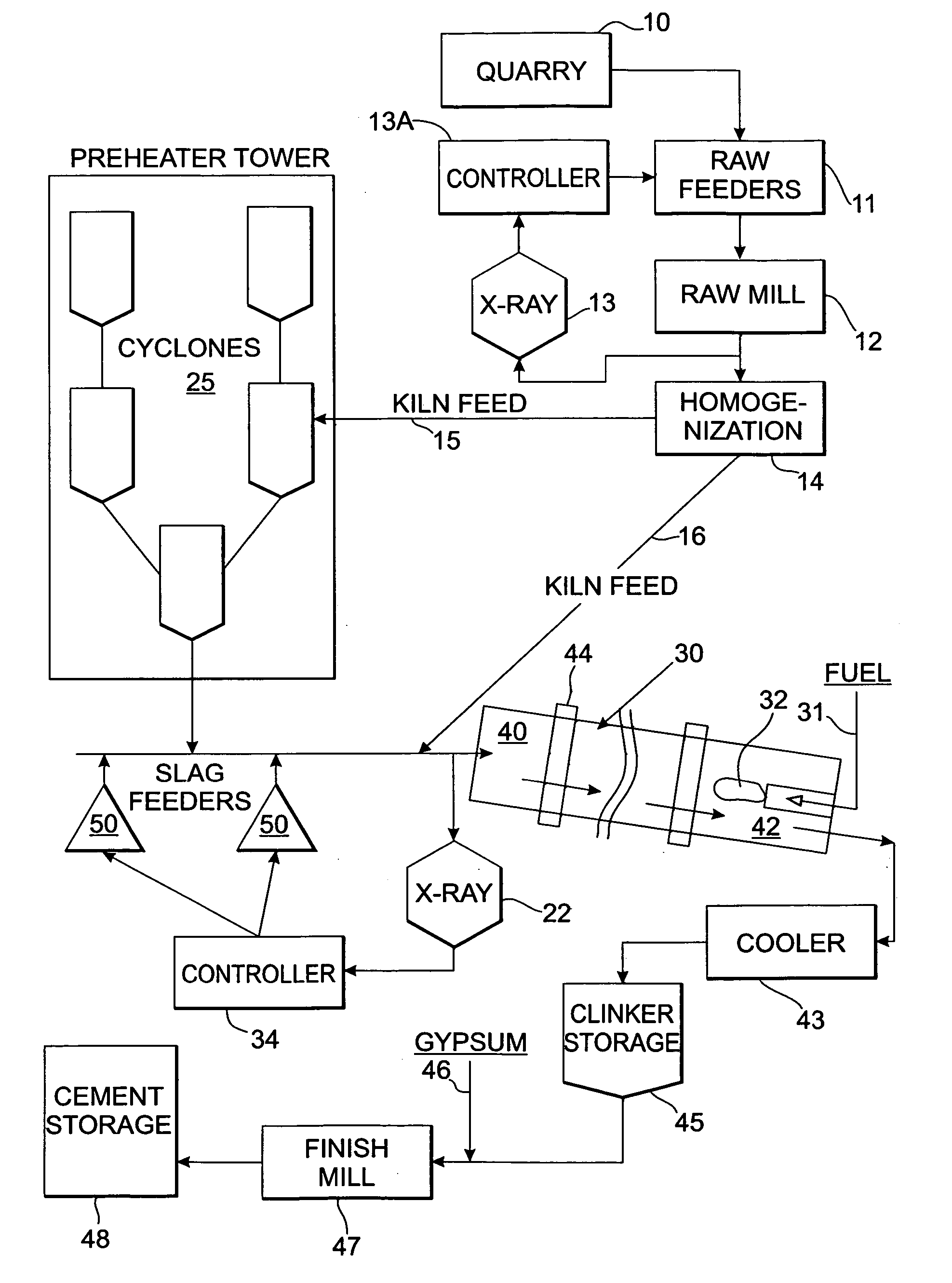
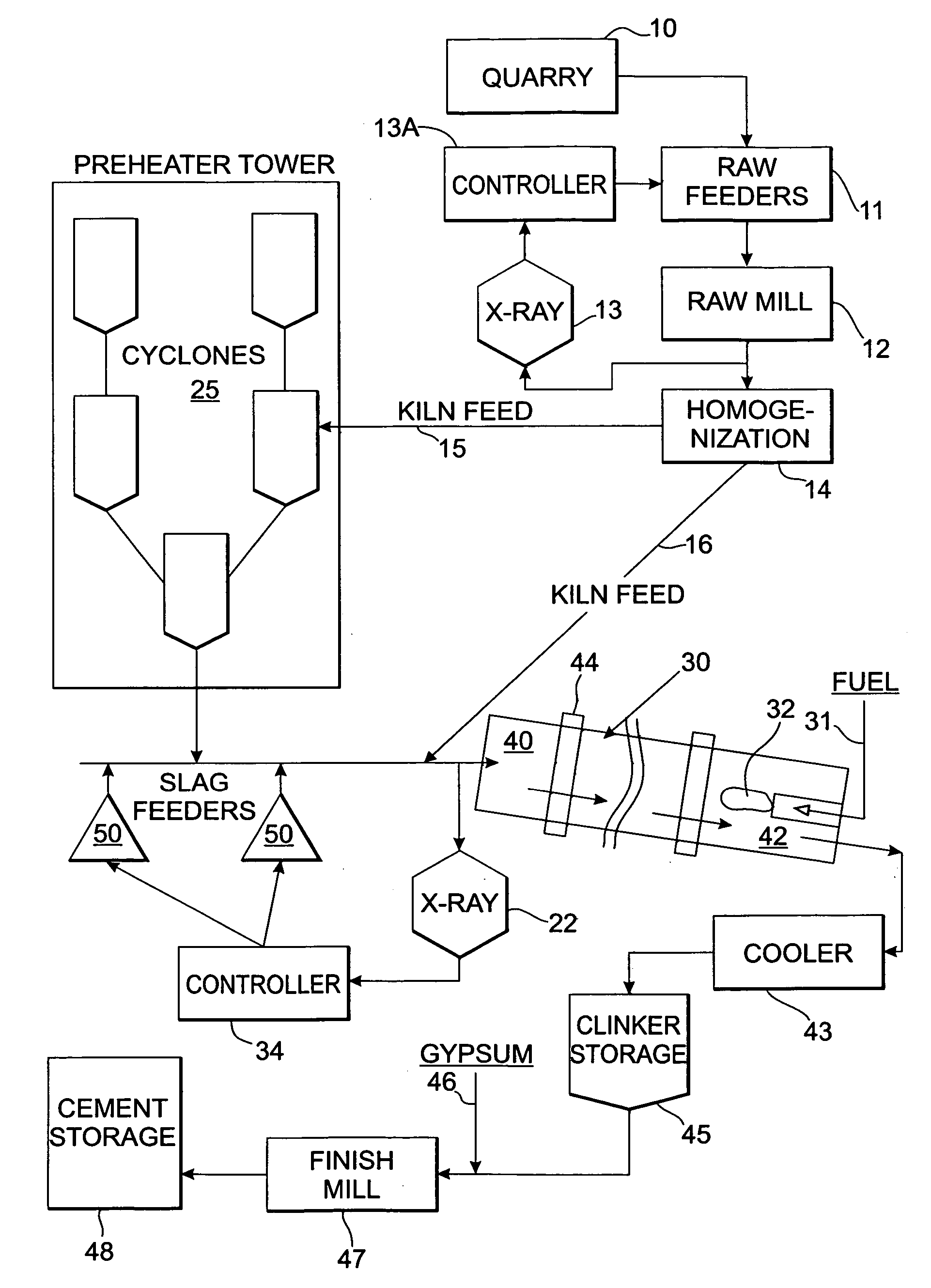
Method and apparatus for control of kiln feed chemistry in cement clinker production
InactiveUS20050132933A1Easy to changeLower the heatTransportation and packagingRotary drum furnacesRefractory wearChemical composition
A method and apparatus for controlling cement clinker production uses a detection device disposed proximate to the feed end of a rotary cement kiln to detect the chemical analysis of a combined additive / kiln feed mixture. A controller changes the feed rate of the additive feeder to adjust for differences between the detected chemical composition and a chemical target specification such as tricalcium silicate, tricalcium aluminate, lime saturation, silica ratio or aluminum to iron ratio. The timely and convenient adjustment of kiln feed chemistry provides more uniform kiln feed chemistry resulting in better kiln operation in terms of productivity, fuel efficiency and less refractory wear. This method and apparatus can provide chemical adjustments for different grades of clinker.
Owner:BLUM BERNARD
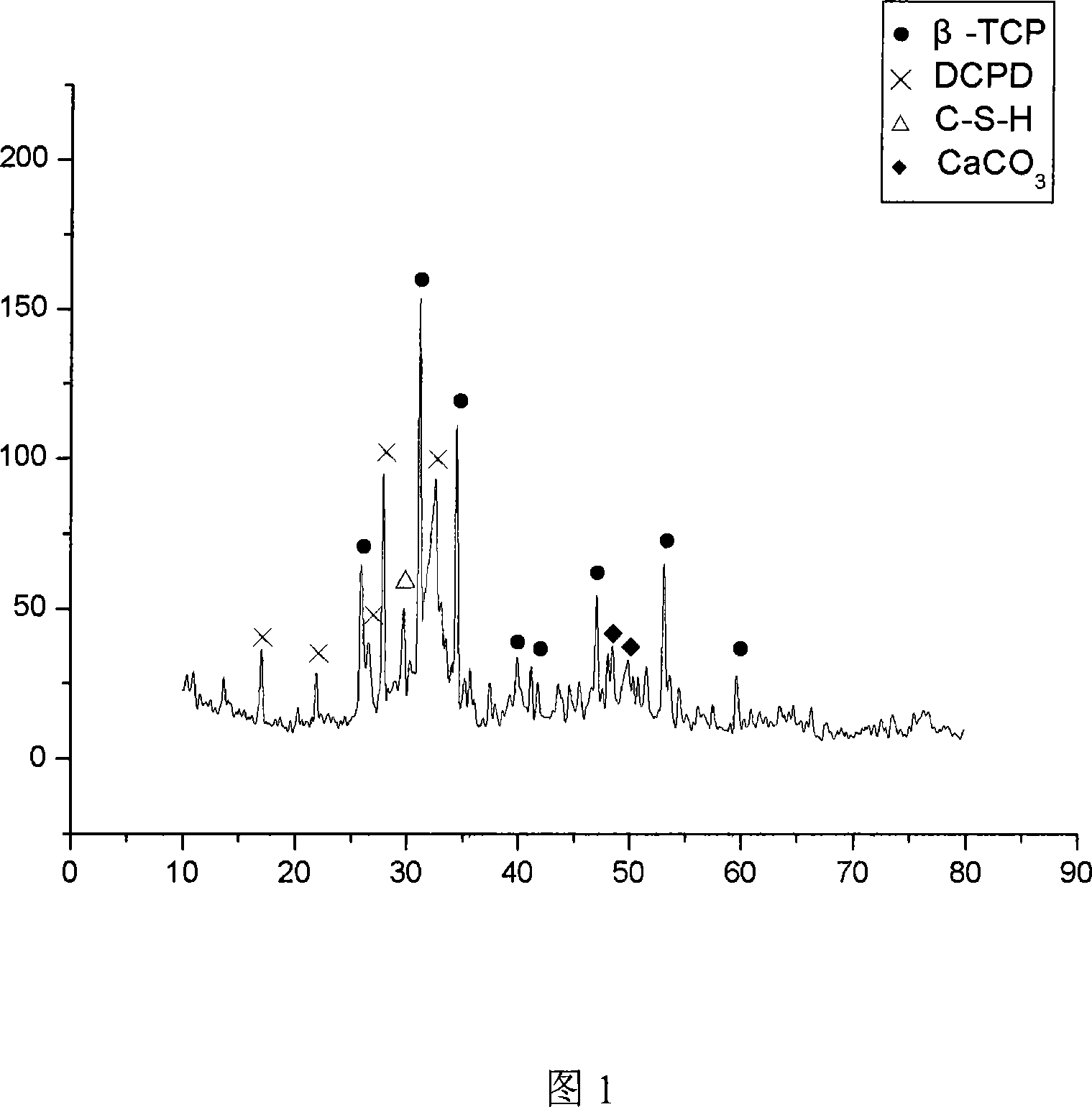
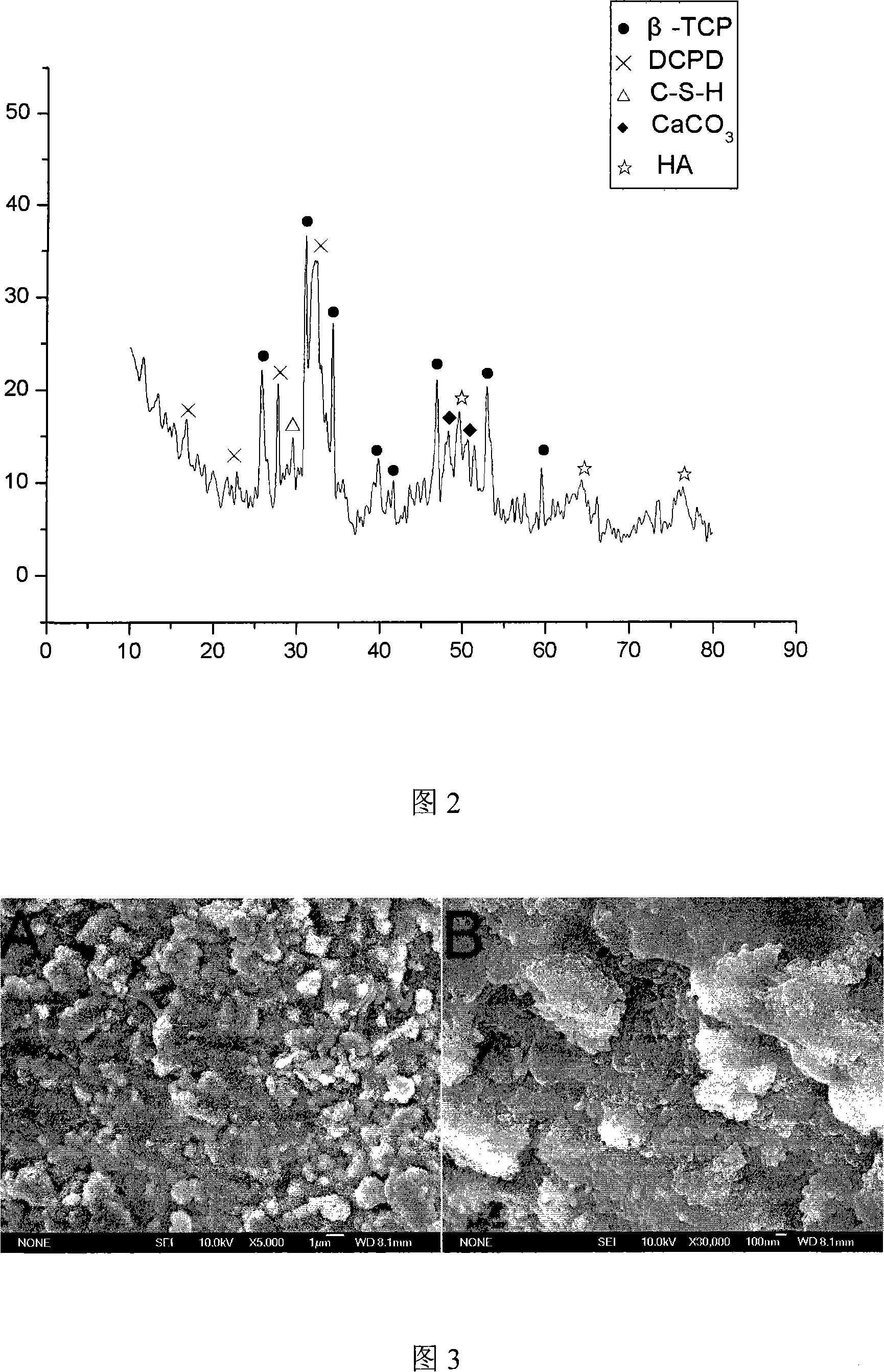
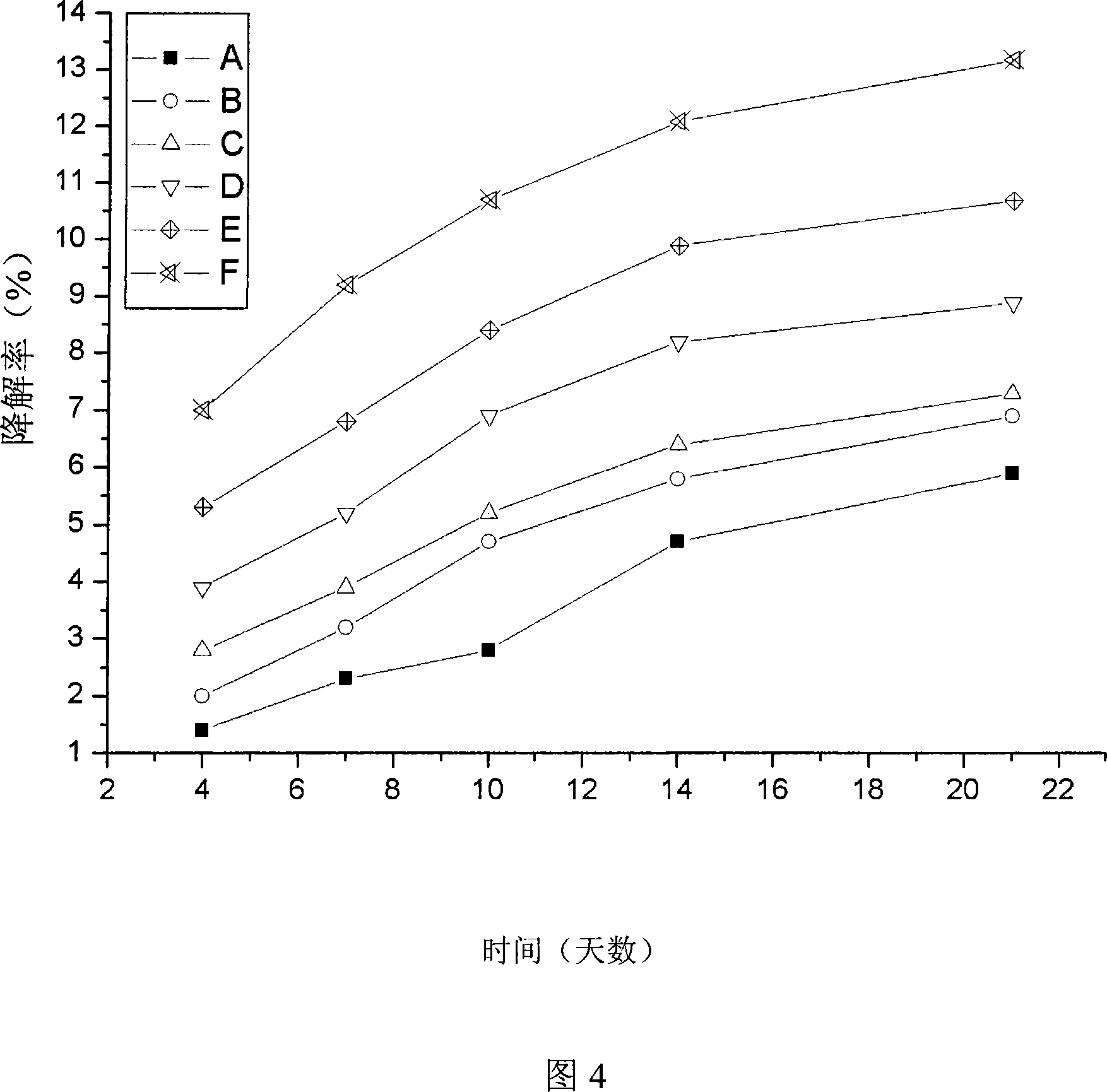
Complex self-curing material, method and application of bioactivity calcium phosphate/tricalcium silicate
InactiveCN101157045AImprove biological activityGood biocompatibilityPhysical/chemical process catalystsCalcium silicateCalcium biphosphate
The invention relates to human bone defect filling original position self-solidified bioactive material and the preparation method thereof. The material is bone / tooth defect repair material which is developed with bioactive calcium phosphate / tricalcium silicate composite powder and mixing liquid having the biological activity as raw materials. Compared with the prior material, the material of the invention has the advantages that the biological activity is excellent, the shaping is arbitrary, the material is self-solidified, no cytotoxicity exists, and the degradation is gradual, the degradation rate can be adjusted, and the effect of inducing bone-like apatite formation can be achieved; the biological activity is more excellent than that of the calcium phosphate calcium phosphate bone-like cement, and the preparation method is simple.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Preparation for producing a material used to restore a mineralised substance, particularly in the dental field
ActiveUS20060102049A1Increase rangeColor matchingImpression capsOther chemical processesChlorideReducing agent
A preparation contains an aqueous liquid part, a solid part comprising at least one silicate selected from tricalcium silicate Ca3SiO5 and dicalcium silicate Ca2SiO4; and calcium chloride CaCl2 and a water reducing agent which are both contained in at least one of the aforementioned parts. According to the invention, the solid part and the liquid part are intended to be mixed in order to obtain the material. The preparation can be used to restore a mineralized substance, particularly in the dental field.
Owner:SEPTODONT SPECIALITES SEPTODONT
Calcium sulfate semihydrate group combined self-curing bio-active material, preparation and application thereof
The invention relates to a self-solidification biological active material of hemigydrate calcium sulfate base, relative production and application. Wherein, the invention is characterized in that: said material is paste formed by harmony liquid, hemigydrate calcium sulfate / tricalcium silicate composite powder, while their mass ratio is 0.8-1.2:1; said harmony liquid is one of deionized water, analogue humor, inorganic salt solution or inorganic solution; the content of tricalcium silicate in the hemigydrate calcium sulfate / tricalcium silicate composite is 1-50%. The invention can be shaped freely, without cell toxicity, while it can be degraded stepped with adjustable speed, and it can induce the generation of skeleton agustite, with higher activity then calcium sulfate material.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
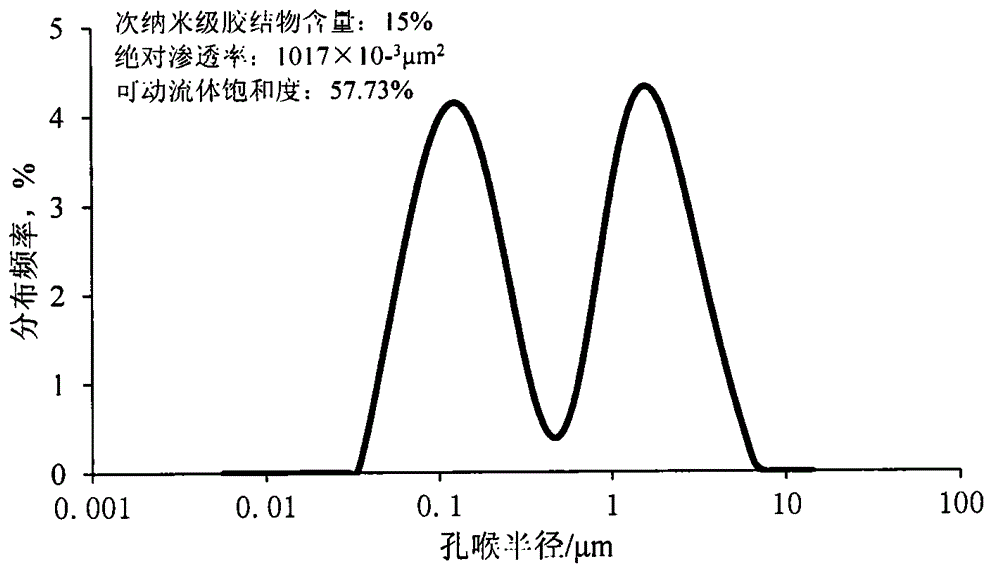
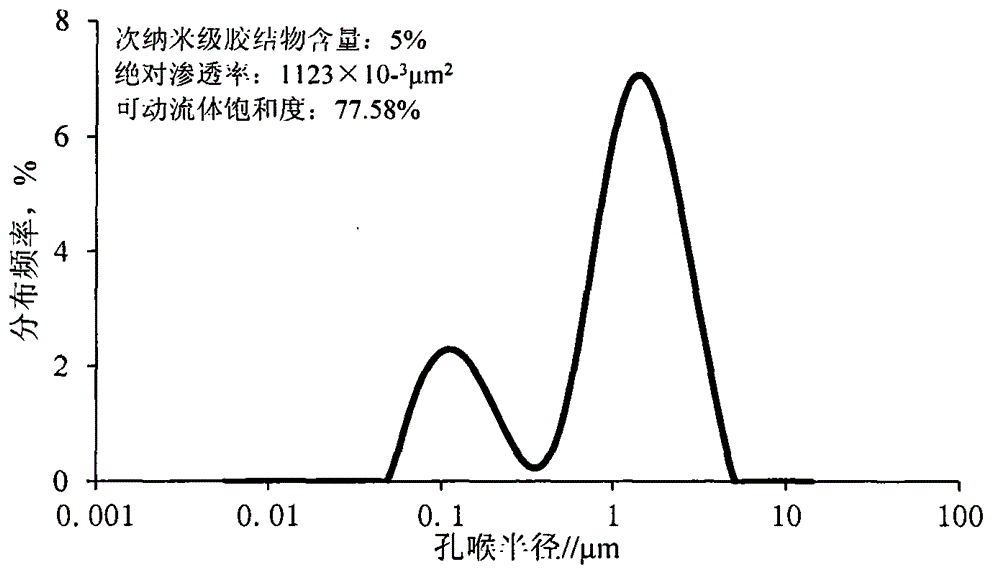
Artificial core containing controllable and movable fluid, and manufacturing method of artificial core
InactiveCN104390825AOvercoming the problem of poor simulation accuracyPrecise pore distributionPreparing sample for investigationEpoxySilicic acid
The invention relates to an artificial core containing controllable and movable fluid, and a manufacturing method of the artificial core. The manufacturing method mainly comprises the following steps: firstly, evenly stirring quartz sand, traditional epoxy resin cement and sub-nanoscale powder cement, wherein the median particle diameter of the sub-nanoscale powder cement is within the range from 0.7 to 1.0mu m, and the sub-nanoscale powder cement is formed by tricalcium silicate, dicalcium silicate, tricalcium aluminate and crystallized calcium sulfate according to the mass ratio of (0.20-0.25) to (0.30-0.35) to (0.25-0.35) to (0.20-0.30); putting the mixture into a cylindrical steel mould, pressurizing, and drying at high temperature to obtain the artificial core. Due to the change of proportions of two types of mixtures, the artificial core has better similarity with the natural core in the macroscopic aspects such as porosity and permeability, and the distribution proportion of the movable fluid is changed within the specific range on the basis that different levels of pores in the artificial core reach the specific proportion; furthermore, the artificial core is higher in accuracy, thus meeting the experimental demands such as oil field three-mining chemical flooding optimization, and microscopic use law.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
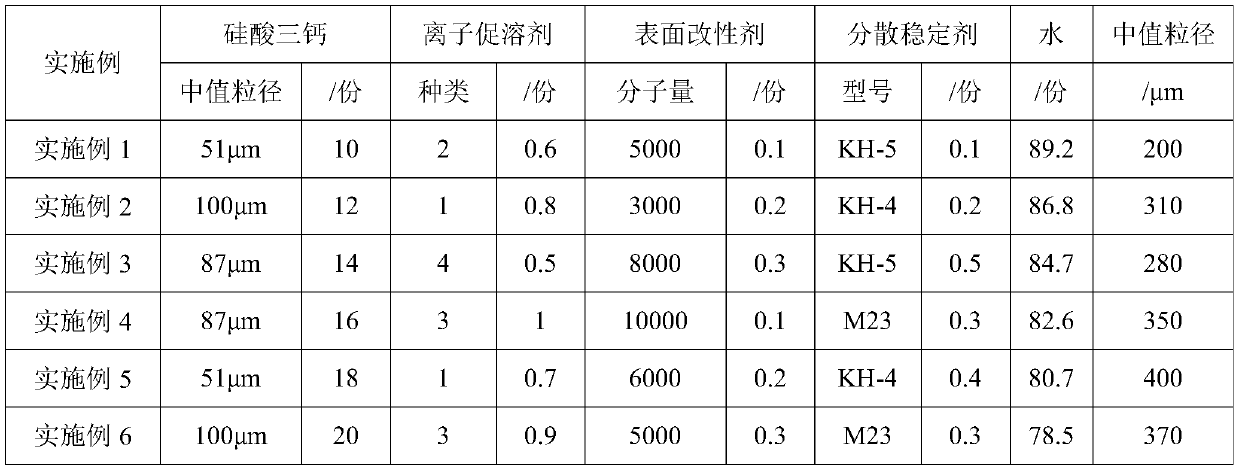
C-S-H gel nanocrystal nucleus early strength agent, preparation method and application thereof
ActiveCN110330257AAccelerated nucleation growthImprove pore structureIon acceleratorsNucleation growth
The invention provides a C-S-H gel nanocrystal nucleus early strength agent, a preparation method and application thereof. The C-S-H gel nanocrystal nucleus early strength agent is mainly prepared from the following components by weight through wet milling process: 10-20 parts of tricalcium silicate, 0.5-1 part of an ion promoter, 0.1-0.3 part of a surface modifier, 0.1-0.5 part of a dispersion stabilizer, and 78.5-89.2 parts of water. The C-S-H gel nanocrystal nucleus early strength agent provided by the invention utilizes the crystal nucleus induction effect of nano form C-S-H gel to accelerate the nucleation growth of hydration products at the early stage of hydration, and can achieve the early strength purpose from the two aspects of by improving the pore structure and inducing nucleation.
Owner:WUHAN UNIV OF TECH
Slow-setting cement for highway roadbeds
ActiveCN102173612AProlong initial setting timeImprove uniformitySolid waste managementSocial benefitsCompressive strength
The invention discloses slow-setting cement for highway roadbeds, which is prepared from the following raw materials in parts by weight: 53 to 60 parts of clinker, 3 to 5 parts of desulfurized gypsum, 1 to 6 parts of desulfurized ash, 15 to 20 parts of limestone, 15 to 25 parts of fly ash, and 0.04 to 0.1 part of slow-setting agent. The clinker mainly contains the following ingredients in percentage by weight: 54 to 62 percent of tricalcium silicate, 15 to 20 percent of dicalcium silicate, 6.7 to l8.7 percent of tricalcium aluminate, and 11 to 13 percent of tetracalcium aluminoferrite. The slow-setting cement provided by the invention is characterized in that: the initial setting time is increased to more than 4 hours, and the final setting time is increased to more than 6 hours; the bending strength (3 days) is more than or equal to 3.6 Mpa, and the compressive strength (3 days) is more than or equal to 17.2 Mpa; the bending strength (28 days) is more than or equal to 6.6 Mpa, and the compressive strength (28 days) is more than or equal to 37.4 Mpa; the performance is better than that of other cement products of the same strength grade and meets the construction requirements for highway roadbeds; and the market prospects are good and the social benefits are increased.
Owner:广州市越堡水泥有限公司
Nano C-S-H gel super-early strength agent and preparation method thereof
The invention relates to a nano C-S-H gel super-early strength agent, which comprises the following components by weight: 30-50 parts of a polyether macromonomer; 3-6 parts of unsaturated acid; 0.5-2parts of an early strength monomer, 0.1-0.3 part of a chain transfer agent; 0.4-1 part of an initiator; and 40-70 parts of water. By strictly controlling the proportion and concentration of a calciumsource and a silicon source, the dosage, concentration and pH value of an added early strength polycarboxylate superplasticizer, the alcohol amine dosage, as well as the rotation speed, temperature and dripping time in the synthesis process, nanosheets of C-S-H with surface adsorption and intercalated structure. The nanosheets reduce the free energy of activation in crystals to zero so as to promote crystal growth and greatly accelerate the hydration of C3S (tricalcium silicate) and C2S (dicalcium silicate) in the Portland cement in concrete, thereby improving the early strength of concrete.
Owner:CHONGQING SANSHENG IND CO LTD
Hydrogen phosphate/tricalcium silicate composite self-curing material with biological activity, preparation and uses thereof
InactiveCN101428153AImprove biological activityGood biocompatibilityImpression capsDentistry preparationsApatitePhosphate
The invention relates to a bioactive dicalcium phosphate / tricalcium silicate compounded self-setting material and the preparation method and application thereof, and belongs to the field of medical biomaterials. The invention is a paste comprising a mixing liquid and bioactive dicalcium phosphate / tricalcium sulfate which are mixed with each other at a mass ratio of 0.5-1.5:1, with the percentage composition of the mass of tricalcium silicate in the composite powder equaling 1-50% and the selected mixing liquid being deionized water, a simulated body fluid, an inorganic salt solution, or an organic matter solution. Compared with the prior materials, the invention has the characteristics of relatively high strength, random moulding, self-setting and biological activity, and can achieve the effect of inducing the generation of bone-like apatite. Moreover, the ratio of mechanical property to biological activity of the material provided by the invention is similar to that of calcium phosphate bone / tooth defect recovery materials.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Preparation method and application method of low-carbon cement clinker
InactiveCN106220008ALower sintering temperatureIncrease the blending ratioCement productionSiliconGypsum
The invention relates to a preparation method and an application method of low-carbon cement clinker. The low-carbon cement clinker is prepared by the steps: uniformly mixing raw materials of silicon-aluminum containing material, limestone and gypsum according to a certain ratio; grinding the mixture to form a raw material, wherein the grain size of the ground raw material requires that residue on a 0.08-mm square hole sieve is less than 10%; calcining the ground raw material for 20 to 40 minutes at the calcining temperature of 1200 to 1300 DEG C; cooling to obtain the low-carbon cement clinker. The low-carbon cement clinker provided by the invention comprises 10 to 25 percent of beta-dicalcium silicate, 5 to 15 percent of calcium sulphosilicate, 15 to 45 percent of tricalcium silicate and 15 to 45 percent of calcium sulphoaluminate. 0.01 to 15 percent of gypsum and 0.01 to 15 percent of limestone are added into the low-carbon cement clinker and then are ground to obtain a cement finished product. The preparation method and the application method of the low-carbon cement clinker have the advantages of low calcining temperature, short coagulation time, high hardening speed, high early strength and continuously enhanced later strength.
Owner:郑州市王楼水泥工业有限公司
Method for preparing high-fixed-carbon-content construction material product
The invention provides a method for preparing a high-fixed-carbon-content construction material product. The method comprises the following steps of: mixing a cementing material which is prepared from dicalcium silicate, tricalcium silicate, calcium hydroxide and other ingredients with water in a certain water-cement ratio; mixing and stirring in a CO2 atmosphere in a certain procedure to absorb a certain amount of CO2 to prepare a construction product blank; and then, performing carbonated curing to absorb CO2 once again to produce the construction material product. According to the method, CO2 is absorbed in the whole process of producing a construction product, so that CO2 can be effectively utilized, the fixed carbon content is improved by 70 percent in comparison with that of single carbonized curing, the traditional mode which has high CO2 emission in construction material product production is broken through, and the product performance can be improved. The method has remarkable effects in relieving the weather problem caused by CO2 emission, shortening production cycle of construction material products and improving production efficiency.
Owner:WUHAN UNIV
Bio-activity tricalcium silicate/semi water calcium sulphate composite self-solidification material, preparation and application
InactiveCN1911458AImprove biological activityGood biocompatibilityProsthesisInorganic saltsSimulated body fluid
A self-solidifying bioactive composition in the form of paste for repairing bone and tooth is proportionally prepared from the mingling liquid chosen from deionized water, simulative body fluid, inorganic salt solution and gelatin, and the composite powder consisting of tricalcium silicate and semi-hydrated calcium sulfate.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Negative electrodes of alkaline batteries and their methods of fabrication
InactiveUS20050244714A1Improve cycle lifeLarge capacityNon-metal conductorsFinal product manufactureTricalcium aluminateMaterials science
The present invention discloses negative electrodes for alkaline storage batteries and their methods of fabrication. The material for said negative electrode comprises of an additive that has at least one calcium compound selected from the following: tricalcium silicate, dicalcium silicate, and tricalcium aluminate. The concentration of said additive is between 1 wt % and 15 wt % of the material of said negative electrode. To fabricate said negative electrode, said additive is mixed with an active material for the negative electrode to form a paste, which is then dried. This method of fabrication is simple, convenient and low in cost. An alkaline battery using said material for its negative electrode has long cycle life and a large capacity.
Owner:BYD AMERICA CORP
Premixed biological hydraulic cement paste composition and using the same
A premixed cement paste for use in medical or dental applications. The premixed cement paste remains fluid when stored in a hermetically sealed condition, but hydrates and hardens to set when placed in a physiological environment. The cement paste includes at least one calcium silicate compound and at least one substantially water-free liquid carrier mixed with the at least one calcium silicate compound; the substantially water-free liquid carrier avoids hydration of the mixture during storage, but undergoes exchange with aqueous physiological solutions so that the cement past hydrates and hardens to set when placed in a physiological environment. The paste may be placed in the physiological environment by injection, for example. The at least one calcium silicate compound may be, for example, calcium silicate, dicalcium silicate, tricalcium silicate, or mixtures thereof. The substantially water-free liquid may be, for example, ethylene glycol, polyethylene glycol, liquid glycerol, glycerin, ethyl alcohol, vegetable oil, animal oil, silicon oil, hydroxypropyl methylcellulose, or mixtures thereof. The substantially water-free liquid carrier preferably includes water in an amount less than about 20% by weight percent of the paste. The paste may include a secondary phase for enhanced properties, such as, for example, a fibrous or particulate material for enhanced mechanical properties, biodegradable or soluble materials that provide room for bone in-growth, bioactive materials such as antibiotics that elute into the physiological environment from the set cement, and radio-opaque materials that enhance X-ray imaging of the cement.
Owner:INNOVATIVE BIOCERAMIX
Water-cured temperature-sensitive injection type dental root canal repair material and application thereof
ActiveCN107334645AWon't change colorEasy to operateImpression capsDentistry preparationsCalcium biphosphateCalcium hydroxide
The invention discloses a water-cured temperature-sensitive injection type dental root canal repair material and application thereof. The water-cured temperature-sensitive injection type dental root canal repair material is white paste, is an injection type root canal repair material and comprises, by mass, 25%-65% of tricalcium silicate or a tricalcium silicate / dicalcium silicate mixture mainly prepared from tricalcium silicate, 2%-10% of calcium hydroxide, 2%-25% of calcium phosphate bone cement, 5%-10% of hydroxybutyl chitosan, 5%-15% of X-ray developing agent, and 18%-28% of an anhydrous nontoxic solvent. Filling of the water-cured temperature-sensitive injection type dental root canal repair material can be achieved in an injected mode, and operation is convenient; and the water-cured temperature-sensitive injection type dental root canal repair material has the good apical sealing ability and biocompatibility and can relieve or even eliminate the root filling reaction. The water-cured temperature-sensitive injection type dental root canal repair material can be used for injection filling treatment of various root canal tip operations.
Owner:武汉新思邦生物科技有限公司
Method for producing cement clinker from paper manufacture waste slag mud
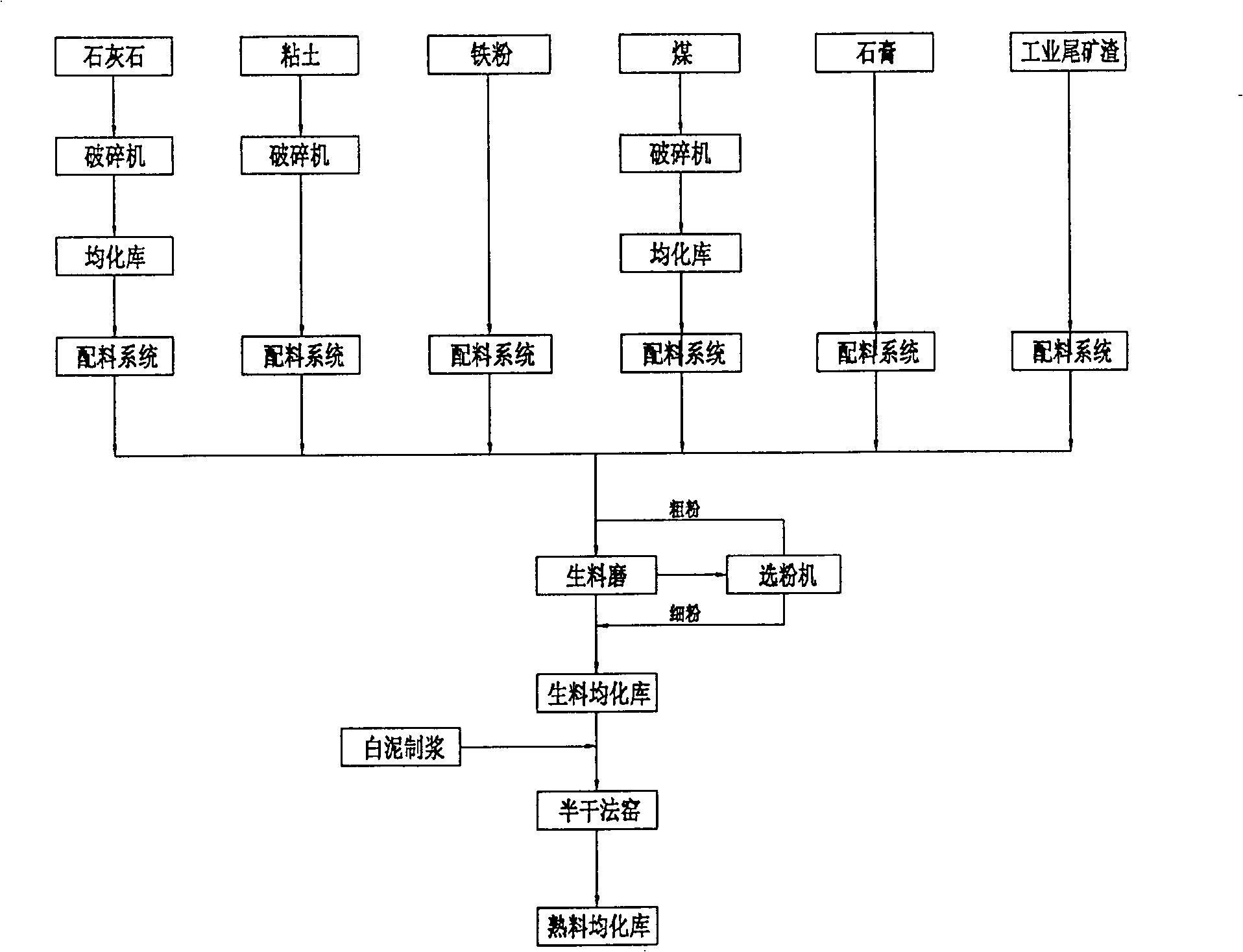
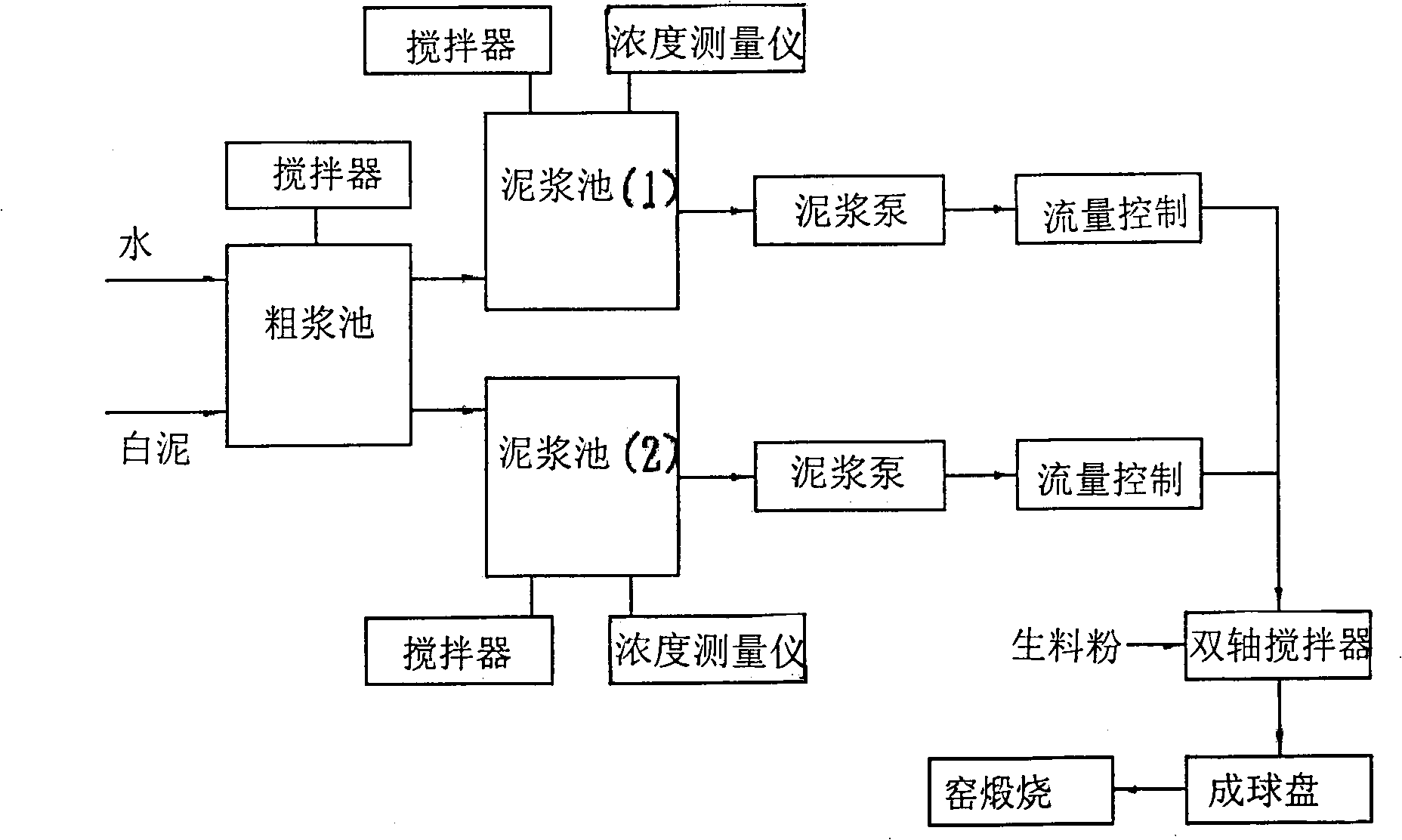
The invention relates to a method for producing cement clinker by using papermaking waste residues and white mud, the process steps are as follows: preparation of raw materials: the consumed raw materials are 1.6 times the clinker; the limestone raw material in the raw materials accounts for 73 percent of the raw materials according to the weight ratio, the limestone raw material is replaced by the white mud, and the white mud accounts for 32.3 percent of the weight of the limestone raw material; the white mud is stirred after the dilution by adding water, then the white mud is sent to a dual-shaft stirring machine via a nozzle by using a high-pressure slurry pump to be mixed with raw material powder, thereby replacing the water for ball shaping, then the mixture is pelletized by a ball shaping disk and then enters a kiln for calcination; the clinker is calcinated; the raw materials are arranged in the cement kiln after the ball shaping for calcination till part of the raw materials are melted, thereby obtaining the silicate cement clinker which takes tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite as main ingredients. The method has the advantages that: the white mud replaces the limestone raw material which accounts for 32.3 percent by weight, thereby solving the problems of the environmental pollution and the land occupation caused by the white mud wastes; the method is simple, the drying is not used, the drying investment is saved, the clinker quality can meet the requirements, and the adaptability to the performances of the raw materials is strong.
Owner:缪建通
Alite-barium calcium sulfur aluminate cement
An Alinty-barium calcium thioaluminate cement contains barium calcium thioaluminate (3-38 wt.%), tricalcium aluminate (3-20), tricalcium silicate (30-60), dicalcium silicate (15-40), and ferroaluminate (3-20). Its advantages are low burning temp, high early strength and hardening speed, and low cost.
Owner:UNIV OF JINAN
Concrete-autolysis-type rapid self-repairing system in water environment and preparing method thereof
The invention relates to a concrete-autolysis-type rapid self-repairing system in the water environment. The concrete-autolysis-type rapid self-repairing system comprises a concrete base body and autolysis-type composite microspheres with which the concrete base body is filled, the surfaces of the autolysis-type composite microspheres are coated with autolysis-type protective films, and complex minerals formed by mixing tricalcium silicate and tricalcium aluminate are arranged in the centers of the autolysis-type composite microspheres. Compared with the prior art, the autolysis-type rapid self-repairing system can be widely applied to the hydraulic concrete structure, the marine concrete structure and the underground concrete structure, self healing of concrete cracked in the water environment can be generated, cost is low, the effect is remarkable, the problems that a traditional repairing method is large in cost and poor in effect are solved, and durability of the concrete is improved.
Owner:TONGJI UNIV
Low-heat anti-cracking portland cement
The invention discloses low-heat anti-cracking Portland cement. The cement clinker of the cement consists of the following minerals in percentage by weight: 40 to 70 percent of dicalcium silicate, 10 to 35 percent of tricalcium silicate, 1 to 4 percent of tricalcium aluminate, 15 to 30 percent of tetra calcium aluminoferrite, and 0.1 to 0.8 percent of free calcium oxide, wherein the content of magnesium oxide in the cement is between 3.5 and 5.0 percent, the content of alkali is not more than 0.55 percent, and the content of sulfur trioxide is not more than 3.5 percent. The cement has the main technical indexes that the ignition loss is less than or equal to 3wt%, the specific area is less than or equal to 340m<2> / kg, the stability is qualified, 3-day tensile strength is not required, the 7-day tensile strength is more than or equal to 13MPa, the 28-day tensile strength is between 42.5MPa and 52.5MPa, the 3-day breaking strength is not required, the 7-day breaking strength is more than or equal to 3.5MPa, the 28-day breaking strength is more than or equal to 7.0MPa, the 3-day hydration heat is less than or equal to 220KJ / kg, and the 7-day tensile strength is less than or equal to 250KJ / kg. The cement can be used for remarkably improving the cracking resistance of hydraulic concrete.
Owner:CHINA THREE GORGES CORPORATION
Cement clinker production method
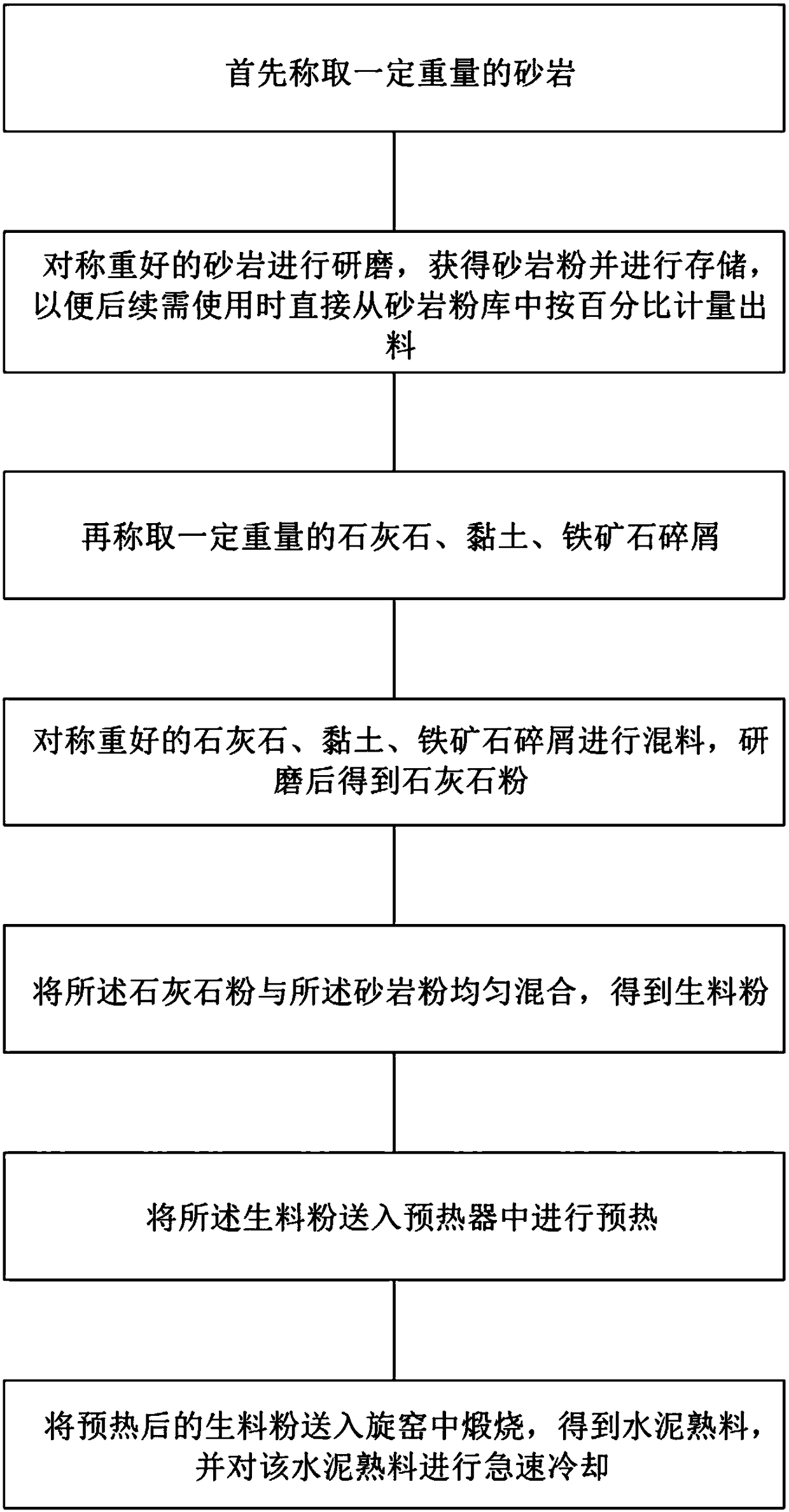
The invention provides a cement clinker production method. The method comprises steps as follows: firstly, a certain weight of sandstone is weighed firstly; the weighed sandstone is ground, and sandstone powder is obtained and stored; a certain weight of limestone, clay and iron ore scraps are weighed, mixed and ground, and limestone powder is obtained; the limestone powder and the sandstone powder are uniformly mixed, and raw powder is obtained; the raw powder is sent into a preheater for preheating; the preheated raw powder is sent into a rotary kiln for calcination, and cement clinker is obtained and subjected to quick chilling. According to the method, the hard sandstone is independently ground, so that reaction activity of quartz crystals in the sandstone powder can be improved, burnability of the raw powder is improved, yield of the clinker is increased, quality of the clinker is improved, electric consumption in the production process is reduced, and production cost is reduced;besides, activity of tricalcium silicate in the cement clinker can be improved, and quality of the cement clinker is guaranteed.
Owner:江西亚东水泥有限公司
Alkali-free liquid setting accelerator and preparation method thereof
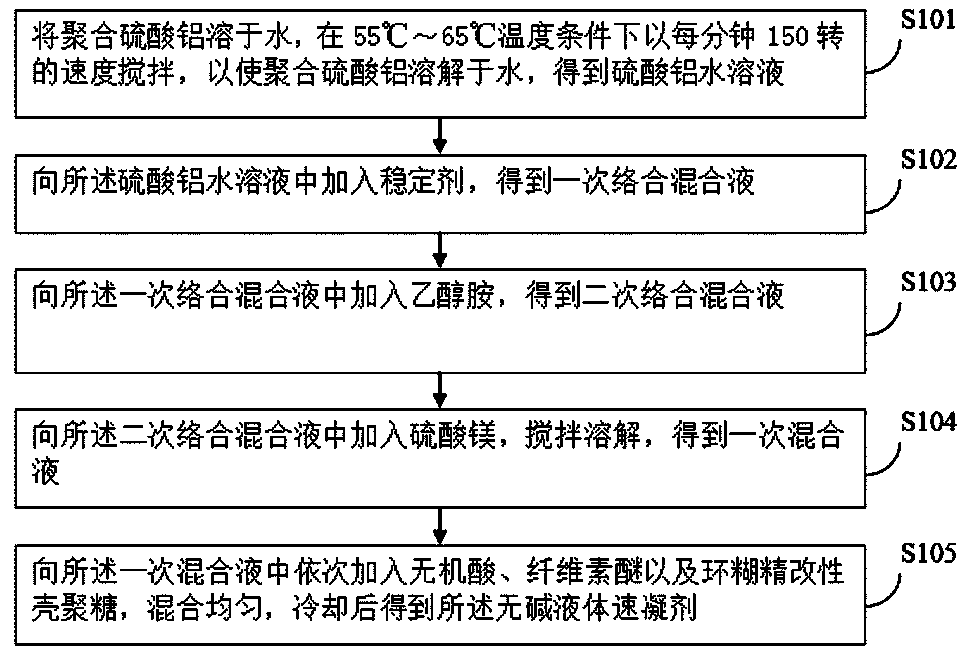
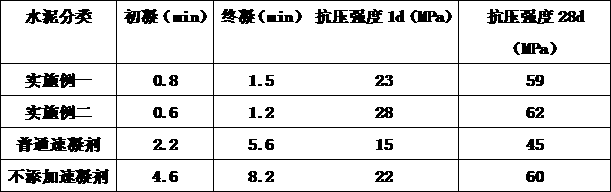
The embodiment of the invention provides an alkali-free liquid setting accelerator and a preparation method thereof. The alkali-free liquid setting accelerator is prepared from polyaluminum sulfate, magnesium sulfate, ethanolamine, inorganic acid, sodium fluoride, cellulose ether, cyclodextrin modified chitosan and a stabilizer. Aluminum ions have a main setting accelerating effect in the settingaccelerator; the alkali-free liquid setting accelerator provided by the invention selects the polyaluminum sulfate; the content of the aluminum ions in the polyaluminum sulfate is relatively high so that the setting accelerating effect can be remarkably improved. Meanwhile, a cement slurry structure doped with the setting accelerator is too rapidly condensed and pores cannot be filled, so that thestrength of the cement slurry structure is damaged; the magnesium sulfate is added and magnesium ions in the magnesium sulfate can be preferably combined with hydroxide ions to generate magnesium hydroxide to provide a crystal nucleus, so that hydration of tricalcium silicate is accelerated to separate out C-S-H gel for filling the pores; the strength of the cement slurry structure can not be damaged at a later period. The alkali-free liquid setting accelerator provided by the invention can be used for preventing a condition that the later-period strength of concrete doped with the alkali-free liquid setting accelerator is reduced.
Owner:山西众诺和建材有限公司
Building material and preparation method thereof
The invention provides a building material and a preparation method thereof, which relates to the technical field of the building material. The building material comprises the following raw materials in parts by weight: 5-7 parts of nano titanium dioxide, 7-13 parts of asphalt, 11-17 parts of bauxitic clay, 8-12 parts of tricalcium silicate, 4-8 parts of polypropylene resin, 6-8 parts of quartz stone, 5-7 parts of calcium acetate, 17-27 parts of polyarmide fiber, 13-21 parts of meerschaum powder, 21-25 parts of bentonite, 15-21 parts of electric furnace slag powder, 11-21 parts of floating beads, 17-23 parts of waste rock wool plate, 15-19 parts of an inorganic adhesive, 0.5-0.9 parts of a foaming agent, 0.3-0.7 parts of a delayed coagulant, 0.2-0.8 parts of a water reducer, and 8-14 parts of water. The preparation method comprises the following steps: 1) weighing the raw materials; 2) milling the materials; 3) heating the materials; 4) roasting the materials; 5) crushing; 6) stirring; and 7) moulding and demoulding, piling and performing maintenance. The method solves the problem that the current environmental protection building materials used for the buildings having high compressive strength, heat insulation, and environmental protection and energy saving requirements are insufficient.
Owner:HEFEI YAKELI NEW BUILDING MATERIAL CO LTD
Quick-hardening earyly-strenthening cement for oil well
The present invention is characterized by that it is formed from mineral components of tricalcium silicate, tricalcium aluminate, sulful trioxide, dicalcium silicate and tetracalcium iron columinate, when the cement is used, the early-strength agent can be added, and when the clinker prepared and roasted according to the requirements for cement composition is used for grinding to prepare cement, the additive can be added. As compared with existent other oil well cemkent said oil well cement has the excellent performances of high early strength, low water loss, slight expansion and short setting time, etc.
Owner:JIAHUA SPECIAL CEMENT
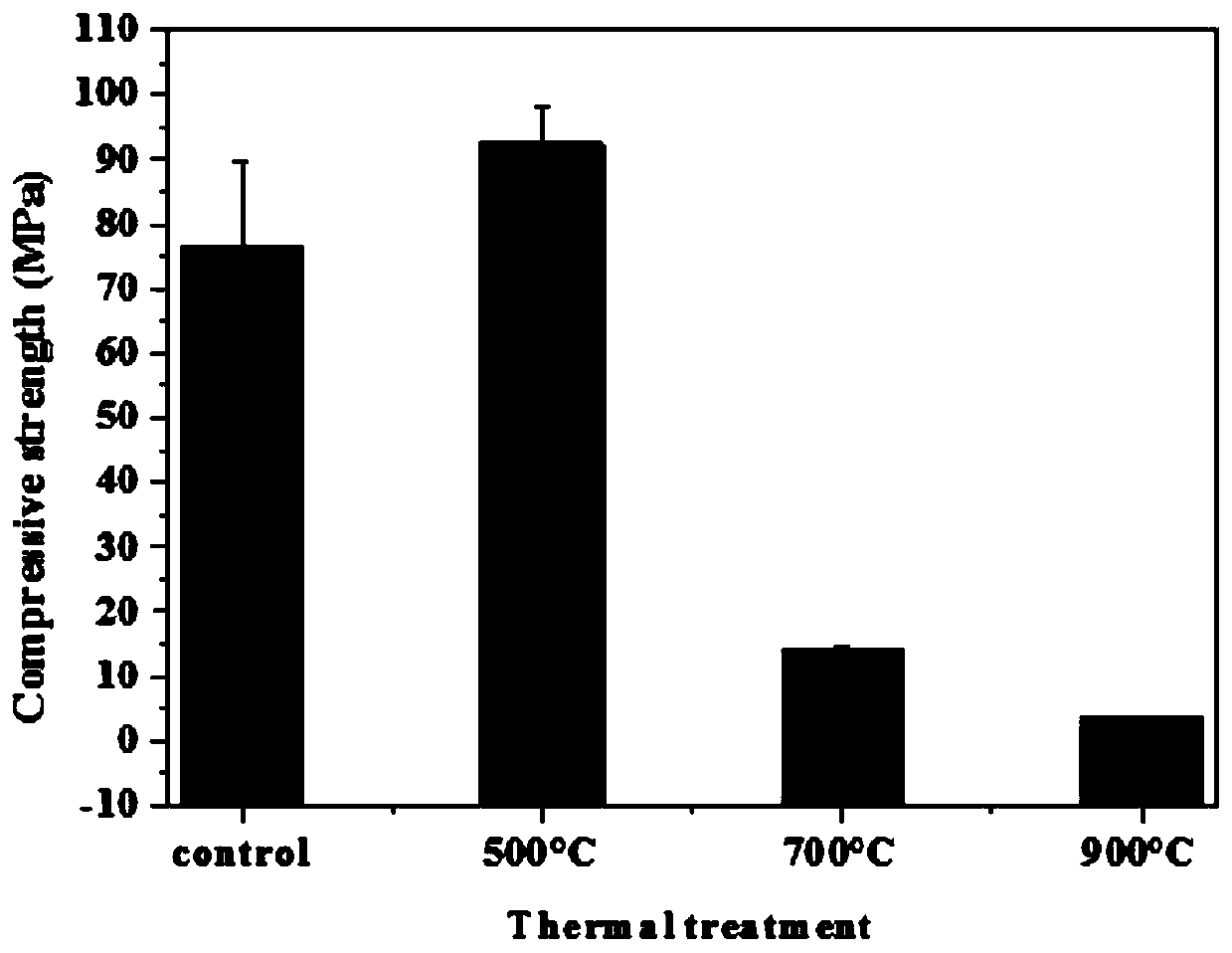
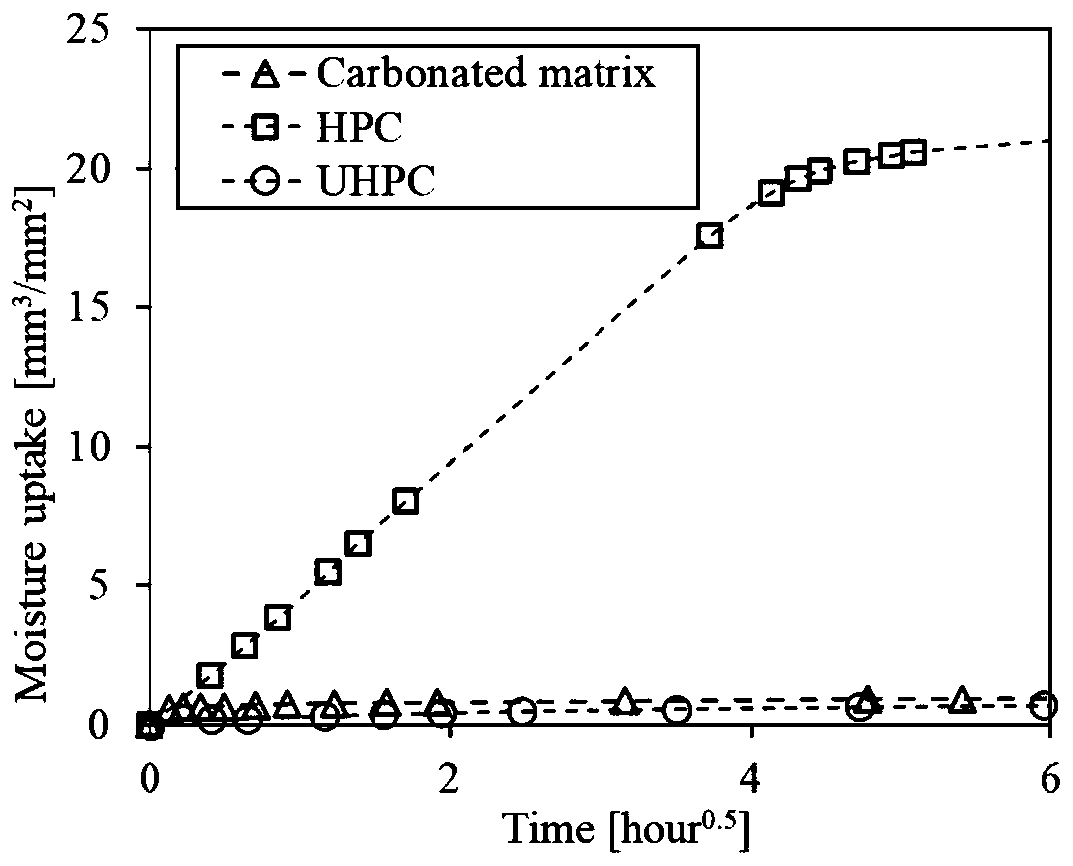
Preparation method of high-performance structural material based on carbonation
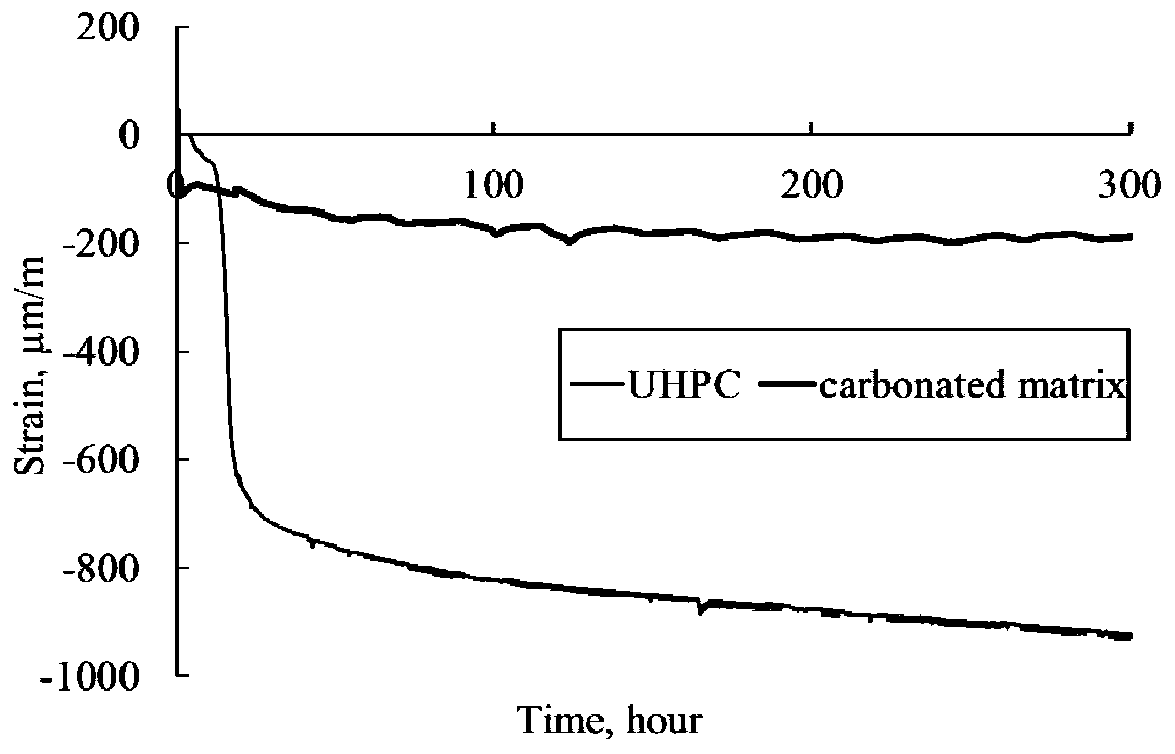
The invention provides a preparation method of a high-performance structural material based on carbonation. According to the preparation method, a calcareous raw material and a siliceous raw materialare fired into a clinker having main minerals including tricalcium silicate, beta-dicalcium silicate and the like which have hydration activity, or gamma-dicalcium silicate, tricalcium disilicate, monocalcium silicate and the like without anhydrous activity, or any combination of the minerals; the clinker is mixed with fine aggregate / and an additive; the mixture is molded at a low water-solid ratio and subjected to carbonation maintenance, and calcium carbonate and silica gel with relatively high mechanical properties are formed in the curing process; compared with C-S-H gel in a cement-basedmaterial, the prepared high-performance structural material has more excellent mechanical properties, thermal stability, corrosion resistance and volume stability, can have shorter maintenance time, and is suitable for engineering construction such as building exterior walls, ocean facilities and rapid repair.
Owner:山东汉博昱洲新材料有限公司
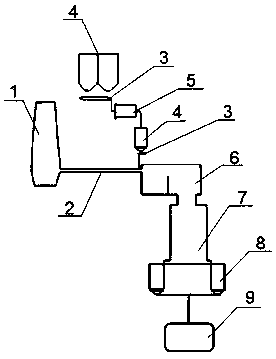
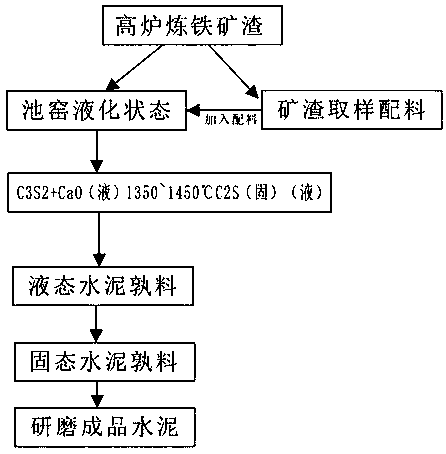
Technology for firing cement clinker by utilizing molten slag liquid phase of blast furnace
The invention relates to a technology for firing a cement clinker by utilizing a molten slag liquid phase of a blast furnace. A device comprises an iron-making blast furnace, a slag tank, a metering belt, a stock bin, a raw mill, a firing tank furnace, a dry quenching slag boiler, a clinker hopper, an intermediate bunker, a liquid feeding port, a melting chamber, a tricalcium silicate crystal chamber, an overflow chamber and a discharging port. The invention has the beneficial effects that the molten liquid slag is directly utilized, the temperature is about 1450 DEG C, the molten liquid slagis an excellent heat source, the sampling chemical test analysis for the molten liquid slag is adopted for supplementing the volume of the chemical element of the cement clinker and the slag is fullymixed and dispersed and reacts under the furnace temperature for keeping liquid state till the main mineral tricalcium silicate is generated; the cement clinker is lastly acquired in the manner of dryquenching, pelletizing and heat exchange in dry quenching slag boiler; the energy consumption of slag cooling and milling is reduced; the energy consumption of preheating, dewatering, decomposing andsintering for forming liquid phase in the present process of producing and firing the cement is reduced; the pollutant emission is reduced; the energy conservation and emission reduction are practically realized; the technology is a practical method for protecting environment.
Owner:上海驰春节能科技有限公司
Method for preparing low-carbon cement clinker by utilizing iron tailings
ActiveCN103373826ARaise the intensity levelReduce intensity levelCement productionCement mortarMaterials science
The invention provides a method for preparing low-carbon cement clinker by utilizing iron tailings. The method for preparing the low-carbon cement clinker comprises the step of firing the iron tailings and limestone as main raw materials at the temperature lower than 1400 DEG C. The low-carbon cement clinker comprises the following mineral compositions: 70-85% of dicalcium silicate, 5-15% of tricalcium silicate, 0-5% of tricalcium aluminate and 4-15% of tetra calcium aluminoferrite. The method has the advantages that the proportion of iron tailings in the raw materials can be up to 32%; in addition, compared with the firing temperature 1450 DEG C of traditional portland cement clinker, the firing temperature is reduced by 50-150 DEG C; compared with the emission of the greenhouse gases in traditional cement production, the emission of CO2 and other NOx-based greenhouse gases is reduced by about 20%. The cement prepared by utilizing the low-carbon cement clinker has the advantages that the 28-day strength can be up to 65-75 MPa; the strength grade is up to 62.5; the cement mortar dispersion degree can be up to 135-200mm; the cement is low in the hydration heat, good in safety and excellent in working performance.
Owner:承德金隅水泥有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com