4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazoline-2-yl)-aminopyrimidine preparation method
A technology of aminopyrimidine and dihydroxypyrimidine, which is applied in the field of preparation of 4,6-dichloro-2-methyl-5-(1-acetyl-2-imidazolin-2-yl) aminopyrimidine, can solve the problem of three Phosphorus oxychloride has the problems of high energy consumption, low yield, high environmental protection pressure, etc., and achieves the effects of low production cost, short reaction time and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
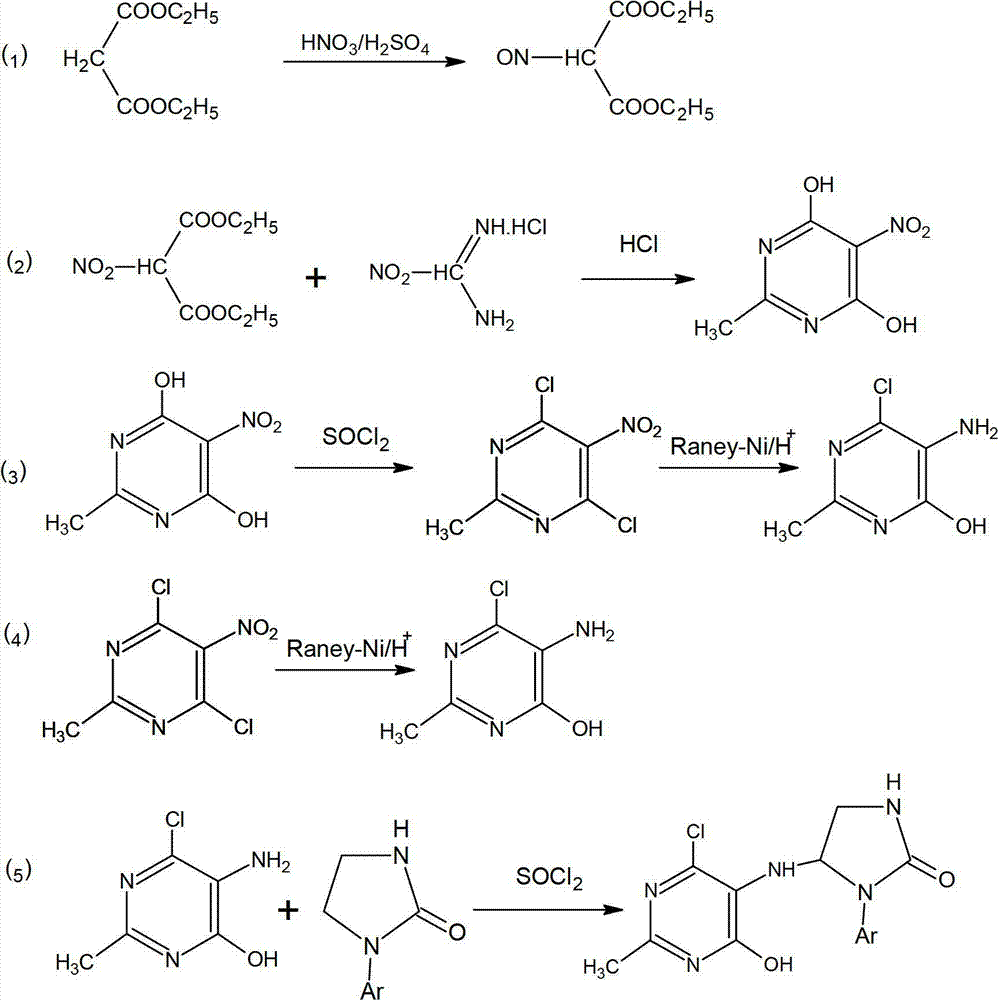
[0022] A preparation method of 4,6-dichloro-2-methyl-5-(1-acetyl-2-imidazolin-2-yl)aminopyrimidine, comprising the following steps in turn:
[0023] (1) Put concentrated nitric acid and concentrated sulfuric acid into diethyl malonate, and carry out nitration at 20-60°C (for example, 20°C, 40°C, 60°C) to obtain 2-nitro-diethyl malonate;
[0024] (2) Under the action of 12N hydrochloric acid, diethyl 2-nitro-malonate is cyclized with acetamidine hydrochloride to synthesize 2-methyl-5-nitro-4,6-dihydroxypyrimidine;
[0025] (3) The 2-methyl-5-nitro-4,6-dihydroxypyrimidine obtained in step (2) is chlorinated with thionyl chloride as the chlorination agent, and the chlorination temperature is controlled at 30-80°C ( Example 30°C, 55°C, 80°C);
[0026] (4) Reduction by Raney nickel hydride;
[0027] (5) Condensing the product obtained in step (4) with acetylimidazolone to obtain product 4, 6-dichloro-2-methyl-5-(1-acetyl-2-imidazolin-2-yl)aminopyrimidine compound.
[0028] Step ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com