Compound containing triple-bond imidazoline, carbon dioxide corrosion inhibitor containing triple-bond imidazoline, and preparation method of carbon dioxide corrosion inhibitor
A technology based on carbon dioxide and imidazoline, applied in chemical instruments and methods, drilling compositions, earthwork drilling and mining, etc., can solve problems such as poor water solubility, layered precipitation, and influence on the use of pharmaceuticals, and achieve good water solubility, good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
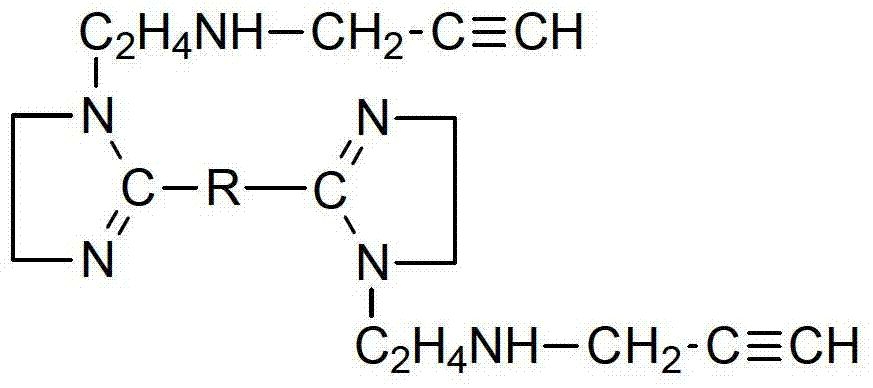
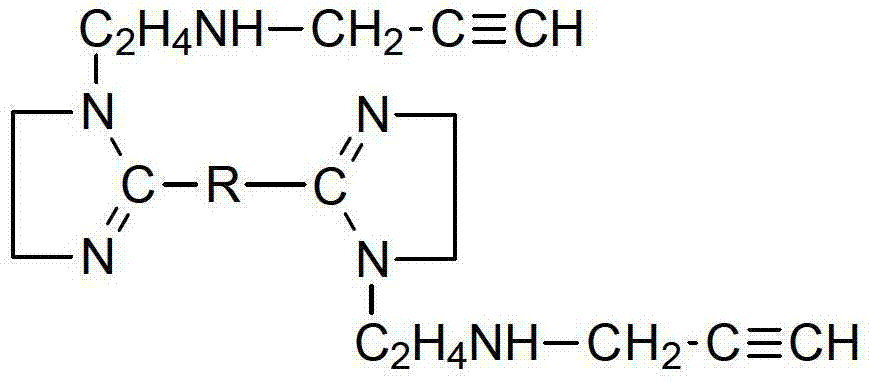
[0032] Add sebacic acid and diethylenetriamine with a molar ratio of 1.0:1.5 into the reactor in a certain order, and raise the temperature to 110°C under stirring conditions. At this time, liquid separates and keeps reflux for 1h; then raise the temperature to 140°C, Reflux for 3 hours for amidation reaction; continue to heat up to 280°C and reflux for 3 hours for cyclization reaction to obtain a bis-imidazoline intermediate;
[0033] Lower the temperature of the bis-imidazoline intermediate to 70°C, add propyne chloride according to the molar ratio of bis-imidazoline intermediate: propyne chloride = 1.0:1.5, then raise the temperature to 100°C, and continue the reaction for 3 hours to obtain a brown-yellow uniform viscous liquid , which is the target product - bis-imidazoline compound containing triple bonds.
[0034] The contents are: bisimidazoline containing triple bonds: 40% by weight, OP-10: 3.0% by weight, ethanol: 57% by weight, mixed with each other, and stirred even...
Embodiment 2
[0036] Add adipic acid and triethylenetetramine with a molar ratio of 1.0:1.7 into the reactor in a certain order, and raise the temperature to 120°C under stirring conditions. At this time, liquid separates, and keep reflux for 1.4h; then raise the temperature to 160°C , reflux for 4h for amidation reaction; continue to heat up to 260°C and reflux for 4.5h for cyclization reaction to obtain a bis-imidazoline intermediate.
[0037] Lower the temperature of the bis-imidazoline intermediate to 85°C, add propyne chloride according to the molar ratio of bis-imidazoline intermediate: propyne chloride = 1.0:1.8, then raise the temperature to 130°C, and continue the reaction for 4 hours to obtain a brown-yellow uniform viscous liquid , which is the target product - bis-imidazoline compound containing triple bonds.
[0038] The contents are: triple-bond-containing bis-imidazoline: 45% by weight, OP-10: 3.5% by weight, methanol: 51.5% by weight, mixed with each other, and stirred evenl...
Embodiment 3
[0040] Add sebacic acid and tetraethylenepentamine with a molar ratio of about 1.0:2.05 into the reactor in a certain order, and raise the temperature to 135°C under stirring conditions. At this time, liquid separates and keeps reflux for 1.8h; °C, reflux for 5 hours for amidation reaction; continue to heat up to 240 °C and reflux for 6 hours for cyclization reaction to obtain a bis-imidazoline intermediate.
[0041] Lower the temperature of the bis-imidazoline intermediate to 90°C, add propyne chloride according to the molar ratio of bis-imidazoline intermediate:propyne chloride=1.0:1.7, then raise the temperature to 145°C, and continue the reaction for 5 hours to obtain a brown-yellow homogeneous viscous The liquid is the target product - bis-imidazoline compound containing triple bonds.
[0042] The contents are: bisimidazoline containing triple bonds: 50% by weight, OP-10: 4.0% by weight, methanol: 46% by weight, mixed with each other, and stirred evenly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com