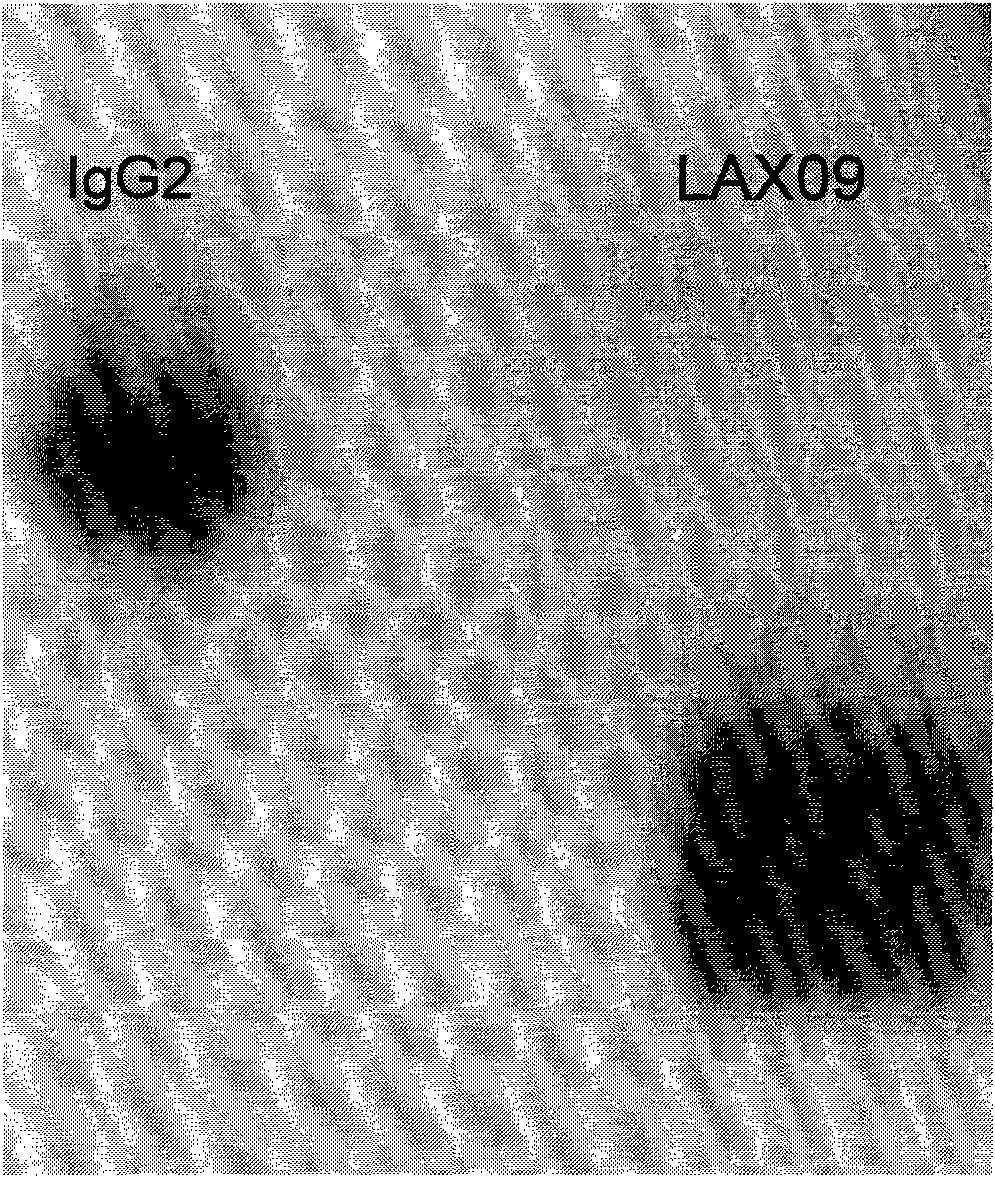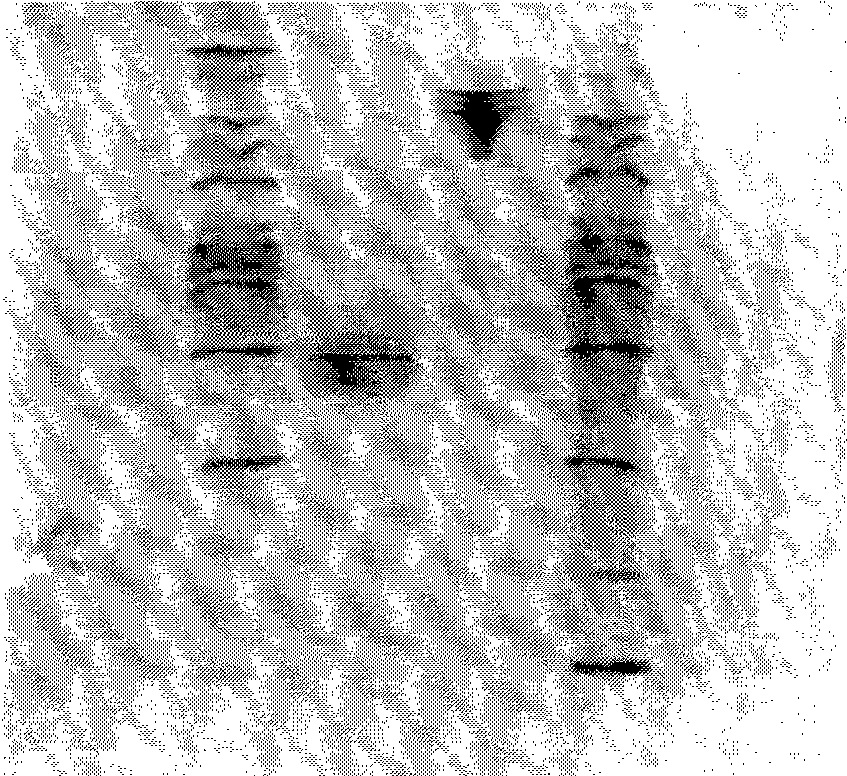Exendin-4 and analog fusion protein thereof
A technology of fusion proteins and analogues, applied in animal/human proteins, fusion polypeptides, drug combinations, etc., can solve the problems of inferior efficacy to exendin-4, short half-life, low binding activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1, exendin-4 fusion protein expression gene synthesis and vector construction
[0098] The DNA construction of the Exendin-4 fusion protein is gene synthesized by ligase chain reaction, and its protein sequence composition is as follows:
[0099] HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPSGGGGAGGGGV
[0100] ECPPCPAPPVAGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVQFN
[0101] WYVDGVEVHNAKTKPREEQFNSTFRVVSVLTVVHQDWLNGKEYKCKV
[0102] SNKGLPAPIEKTISKTKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYP
[0103] SDIAVEWESNGQPENNYKTTPPMLLDSDGSFFLYSKLTVDKSRWQQGNVF
[0104] SCSVMHEALHNHYTQKSLSLSPGK* [SEQ ID NO: 4]
[0105] The fusion protein contains a vector encoding exendin-4 and human IgG2-Fc fusion protein. The IgG2-Fc region contains the CH2 and CH3 portions of the IgG2 constant heavy chain. The IgG secretion leader peptide sequence SP-IgGH was fused to exendin-4 in order to direct the secretion of the synthetic fusion protein into the culture medium. The cDNA encoding the SP-IgGH / e...
Embodiment 2
[0106] Example 2, exendin-4 fusion protein engineering cell line construction, expression and purification
[0107] 1. Construction of exendin-4 fusion protein engineering cell line
[0108] Chinese hamster ovary cells (CHO) were cultured in DMEM (purchased from Invitrogen) complete culture medium containing 10% (volume percentage) fetal calf serum (FCS), and evenly spread on 6-well plates one day before transfection, 3× per well. 10 5 . For the transfection method, refer to the instructions of LIPOFECTAMINE 2000. After 48 hours of transfection, the cells were placed in selective medium (MSX 25uM) and cultured under pressure for about a week. The empty cells basically died, and the surviving cells were seeded in 96-well plates (50 cells / well) and continued to be cultured under pressure. After the cells grew into clones, ELISA was used to detect the expression level of the protein in the culture supernatant, and the wells with high expression were selected and transferred to...
Embodiment 3
[0111] Example 3 Structural analysis of exendin-4 fusion protein
[0112] 1. Western Blot analysis
[0113] After the purified fusion protein was subjected to non-reduced SDS electrophoresis, the electrophoresis band was transferred to methanol-activated PVDF membrane (current: 25mA, time: 2h) using a membrane transfer device (GE). Block the PVDF membrane in 5% skimmed milk for 2 hours, then add the diluted alkaline phosphatase-labeled anti-IgG2 antibody to incubate for 1 hour, wash the membrane with TBST for 1 hour, replace with fresh TBST every 5 minutes, and add CDP after washing -star luminescent detection substrate, press film exposure and development. see attached results figure 2 , the fusion protein was positive for IgG2 antibodies.
[0114] 2. Isoelectric focusing electrophoresis analysis
[0115] The purified fusion protein was analyzed by isoelectric focusing electrophoresis using a Phast System (GE), and a precast gel with pH 3-10 was selected. After the focus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com