Foamless levo oxiracetam injection and preparation method thereof
A technology for oxiracetam and injection, which is applied to the field of non-foaming levooxiracetam injection and its preparation, can solve the problems of obvious pain, reduced product yield, poor patient compliance, etc. High product yield and good patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
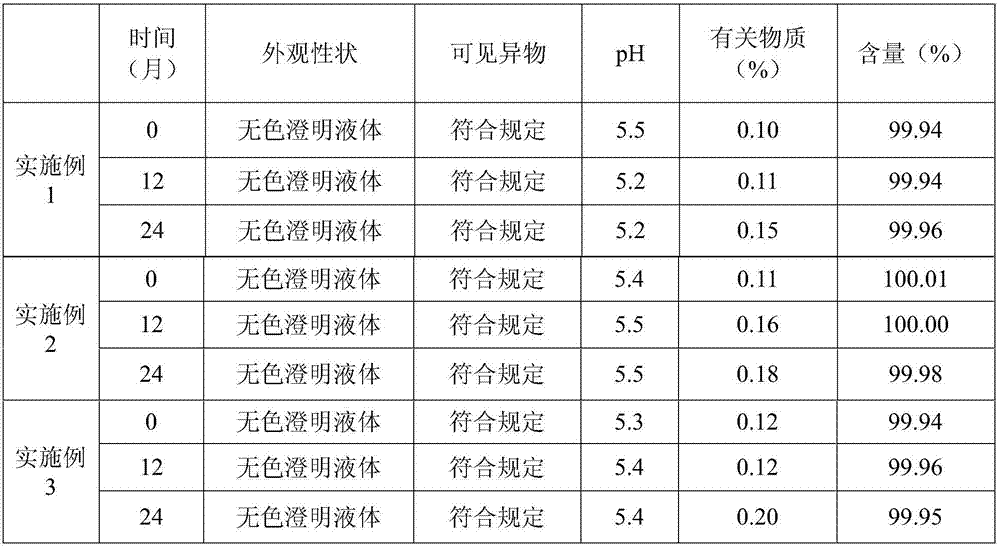
Embodiment 1
[0020] The prescription of the levoxiracetam injection of embodiment 1 is shown in the table below:
[0021] prescription
Dosage
Levoxiracetam
200g
0.05g
4.5g
13g
1.5g
Water for Injection
Add to 1000mL
[0022] Makes 200 bottles
[0023] The preparation method of the levoxiracetam injection of embodiment 1, comprises the following steps:
[0024] (1) Concentrated preparation: take by weighing the prescribed amount of methylparaben and meglumine and add it to water for injection, stir and dissolve, adjust the pH value to 6.0 with 0.1mol / L hydrochloric acid solution, add the prescribed amount of edetate disodium , benzyl alcohol and levo-oxiracetam were stirred and dissolved, and then adjusted with 0.1mol / L hydrochloric acid solution to adjust the pH value to 5.0 to obtain a concentrated solution; the process of concentrated solution needs to ...
Embodiment 2
[0033] The prescription of the levoxiracetam injection of embodiment 2 is shown in the table below:
[0034] prescription
Dosage
Levoxiracetam
180g
0.01g
4g
meglumine
10g
1g
Water for Injection
Add to 1000mL
[0035] Makes 200 bottles
[0036] The preparation method of the levoxiracetam injection of embodiment 2 is the same as that of embodiment 1.
Embodiment 3
[0042] The prescription of the levoxiracetam injection of embodiment 3 is shown in the table below:
[0043] prescription
Dosage
Levoxiracetam
220g
0.1g
5g
meglumine
16g
3g
Water for Injection
Add to 1000mL
[0044] Makes 200 bottles
[0045] The preparation method of the levoxiracetam injection of embodiment 3 is the same as that of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com