Motherwort injection
A technology of injection and motherwort, applied in the field of high-quality motherwort injection, to achieve the effect of less particles, improved performance index and improved quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 Preparation of Motherwort Injection
[0061] Take motherwort and add water to decoct three times, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.36-1.38 (80°C), add water to dilute to a water content of about 35%, and use 88%-95% ethanol respectively Extract three times, stand still, filter, recover ethanol, add activated carbon (consumption is 0.1%) and sterilized activated talc: diatomite (1:1) adsorbent (consumption is 1%) mixture decolorization After the solution is colorless or light yellow and stored for 24 hours, add water for injection to make 1ml contain about 20mg of total alkaloids, decarburize and adsorbent, fine filter, potting, and sterilize to obtain Motherwort Injection. According to spectrophotometry (appendix VB), measure at D525nm wavelength place, biological total alkaloid content is 20mg / ml in terms of stachydrine hydrochloride, adopts the HPLC method measurement that provid...
Embodiment 2
[0068] Example 2 Preparation of Motherwort Injection
[0069] Take motherwort and add water to decoct three times, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.36-1.38 (80°C), add water to dilute to a water content of about 35%, and use 88%-95% ethanol respectively Extract three times, stand still, filter, recover ethanol, add activated carbon (consumption is 0.5%) and contain sterilized activated talc: diatomite (1:1) adsorbent (consumption is 3%) mixture liquid Decolorize until the solution is colorless or light yellow, and store it for 24 hours, add water for injection to make 1ml contain about 20mg of total alkaloids, decarburize and adsorbent, filter finely, potting, and sterilize to obtain Motherwort Injection. According to spectrophotometry (appendix VB), measure at D525nm wavelength place, biological total alkaloid content is 20mg / ml in terms of stachydrine hydrochloride, adopts the HPLC method measuremen...
Embodiment 3
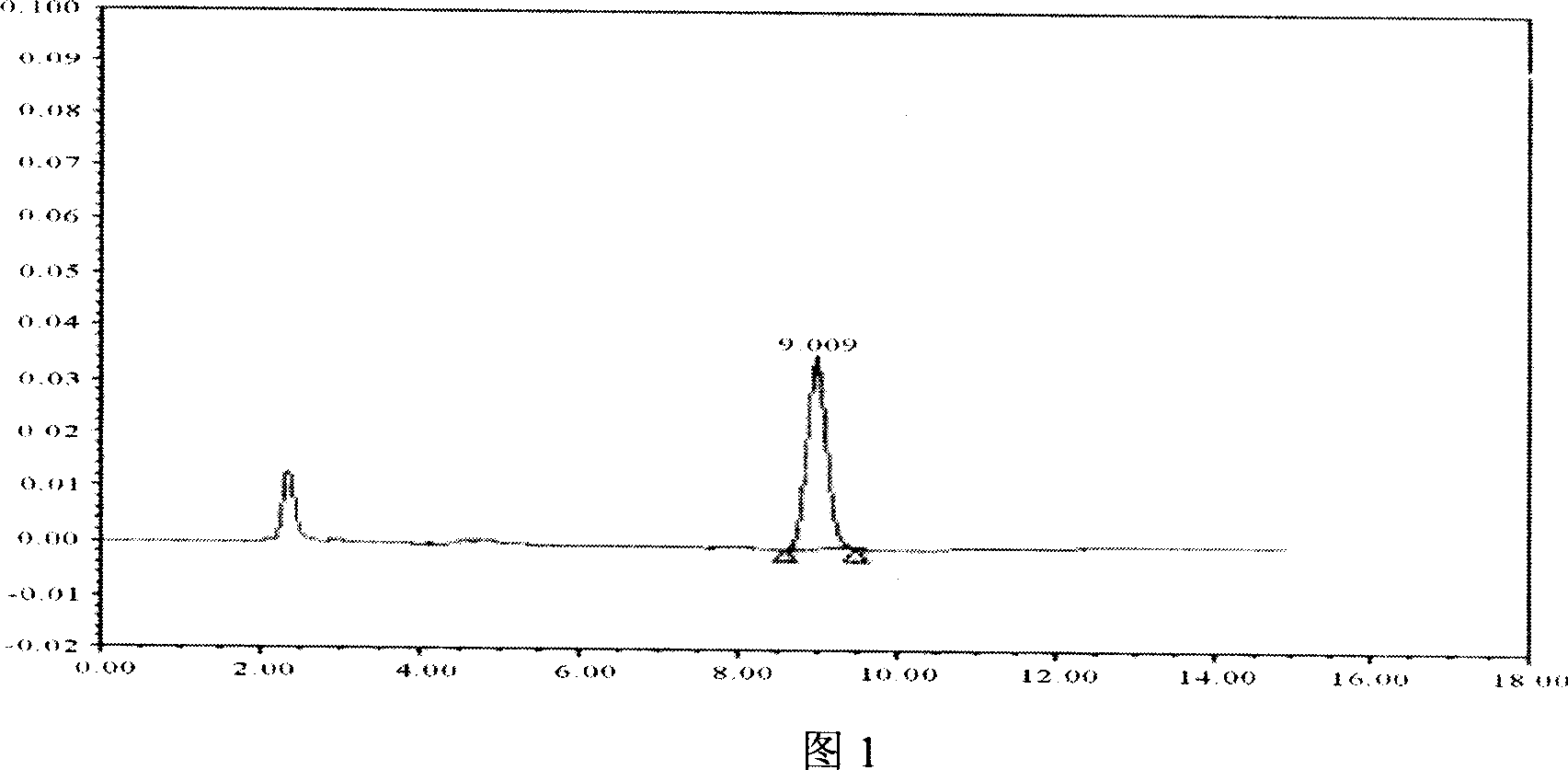
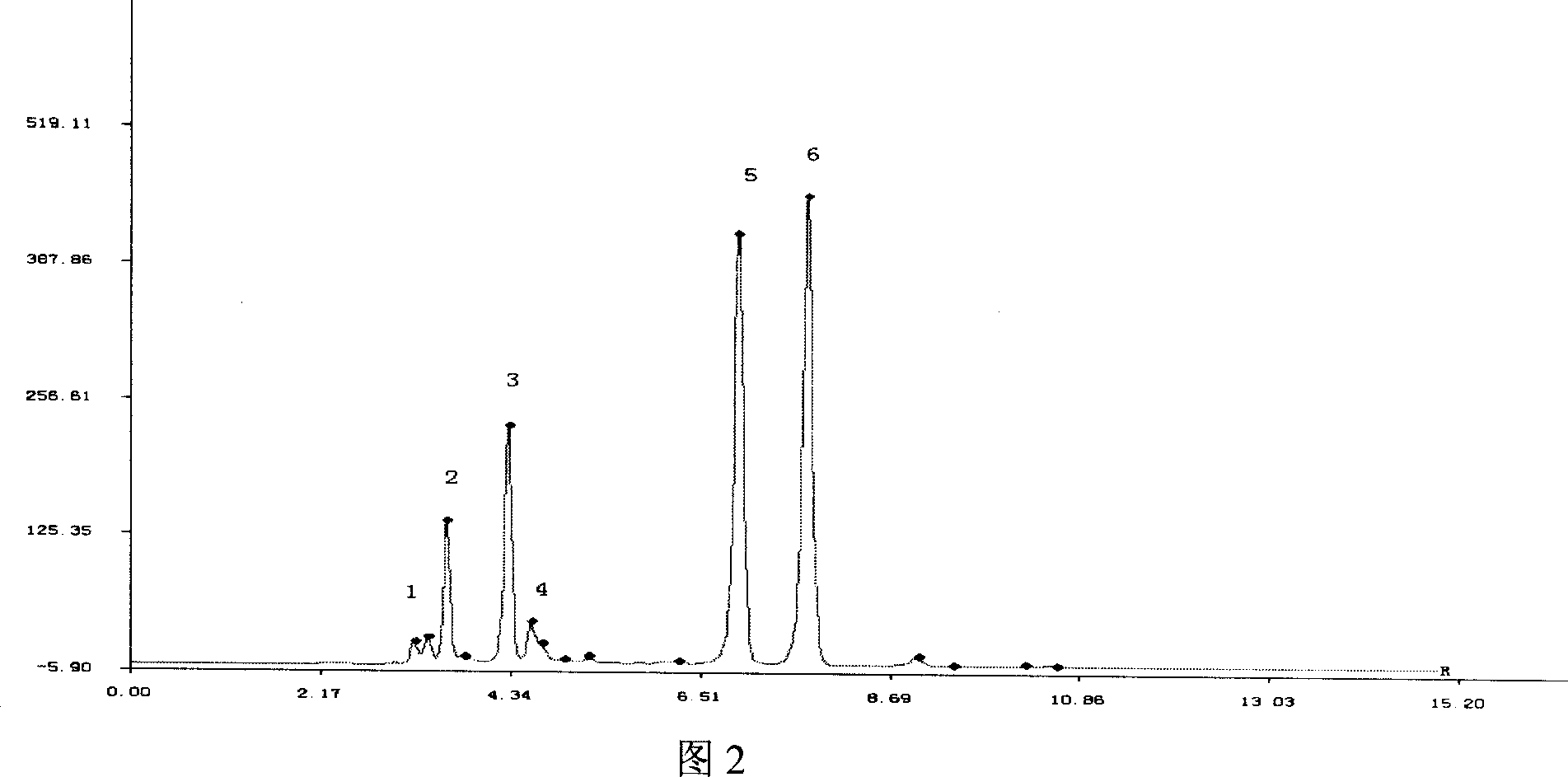
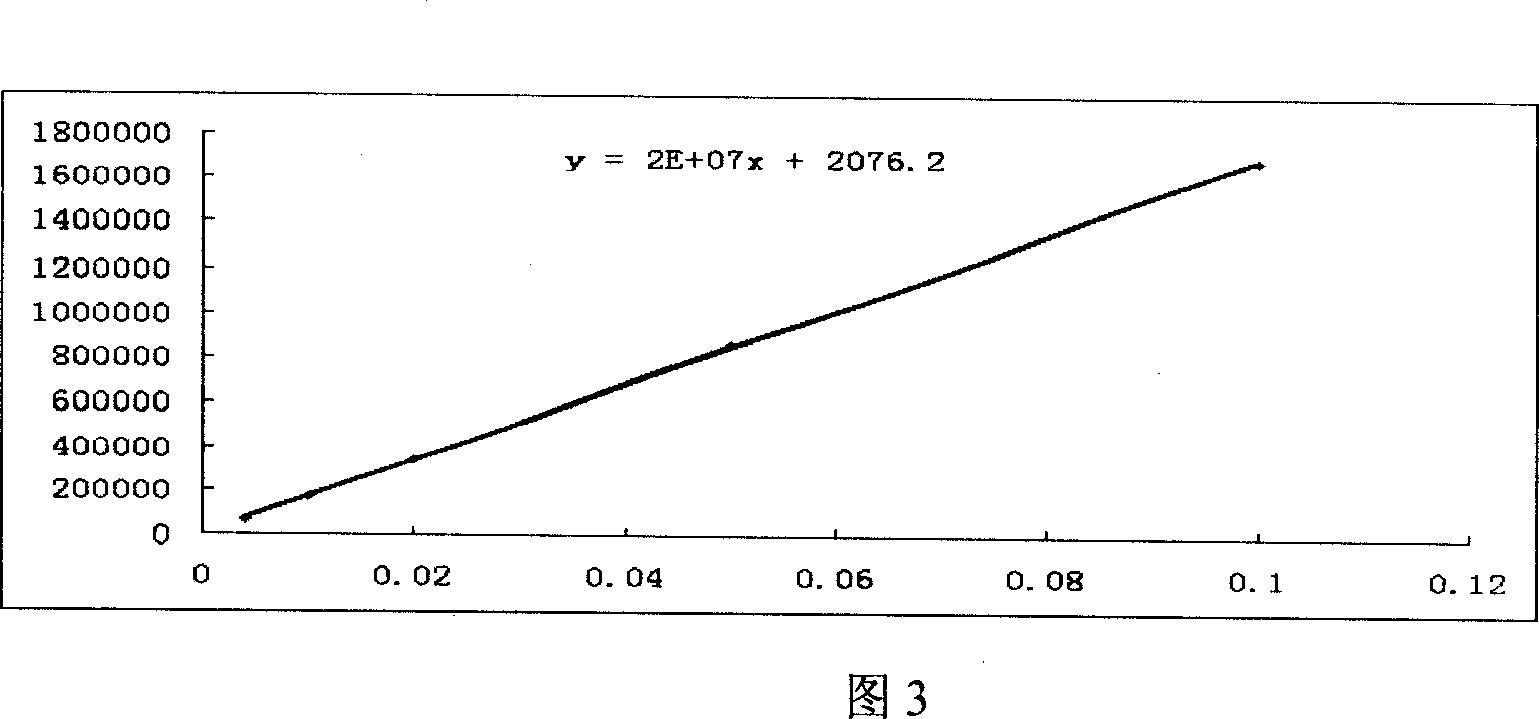
[0076] Example 3 HPLC detection of motherwort injection stachydrine hydrochloride
[0077] (1) Experimental instruments and reagents
[0078] (1) Instrument:
[0079] Waters717-Waters2487-M32 workstation
[0080] Waters2690-Waters2487-M32 Workstation
[0081] Chromatographic column: Alltech SCX 5μ (4.6×250mm)
[0082] Phenosphere SCX 5μ (4.6×250mm)
[0083] MILLIPORE Ultrapure Water Preparation Apparatus
[0084] (2) test drug
[0085] The samples were provided by Chengdu Times First Drug Research Institute Co., Ltd.
[0086] Reference substance National Institute for the Control of Pharmaceutical and Biological Products (Stahydrine Hydrochloride, batch number: 712-9903, for content determination)
[0087]Reagent Potassium dihydrogen phosphate was produced by Chengdu Chemical Reagent Factory, water was ultrapure water, and other reagents were of analytical grade.
[0088] (2) Chromatographic conditions
[0089] (1) Detection wavelength: 203nm.
[0090] (2) Analytical...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com