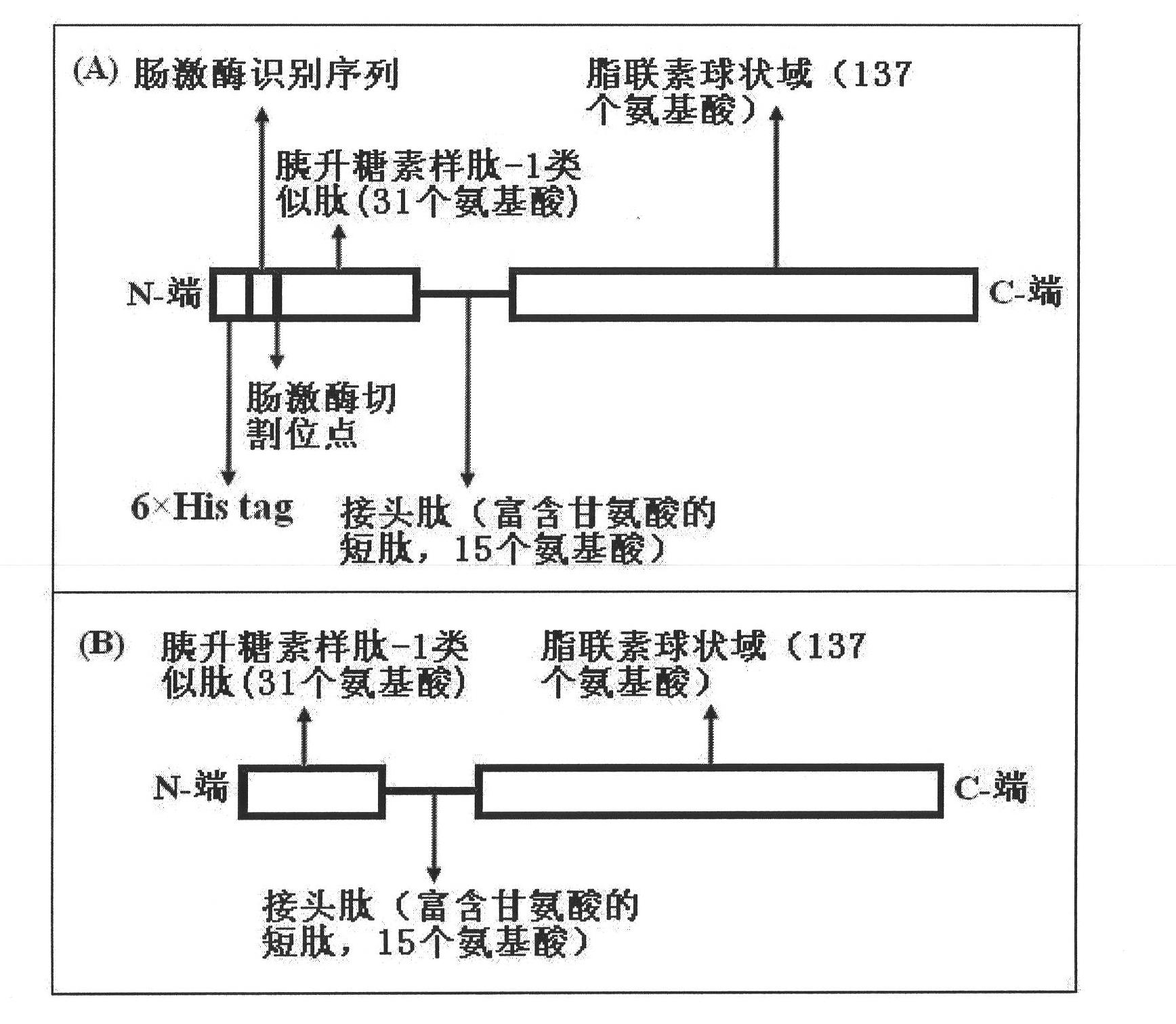
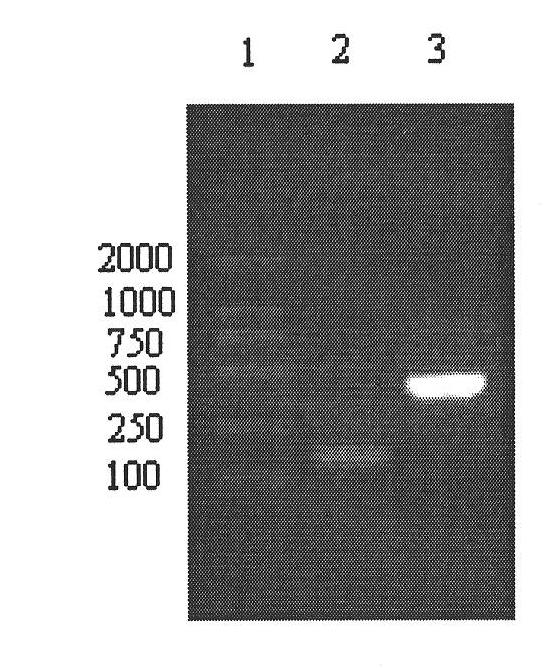
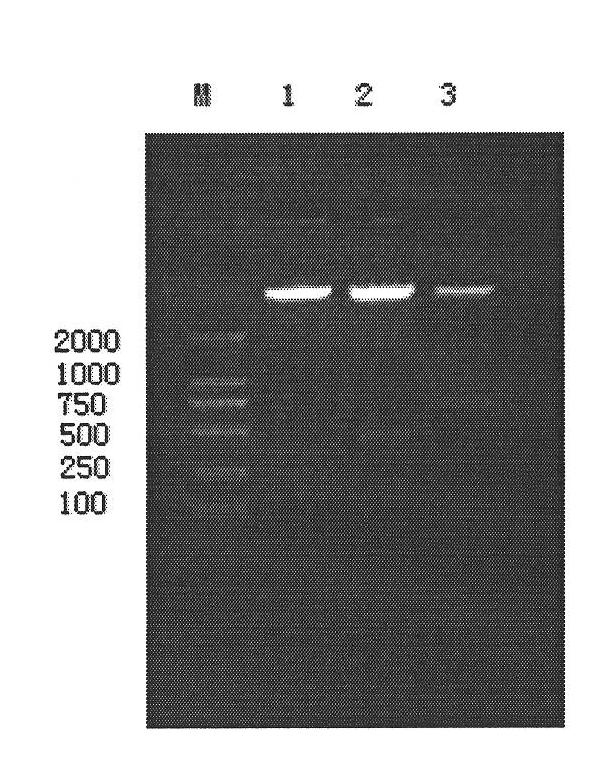
Expression method and application of fusion protein
A fusion protein and spherical technology, applied in the fields of peptide/protein components, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve the problem that the GLP-1 clearance mechanism cannot be explained, the half-life in vivo has not been correspondingly improved, and in vitro Half-life extension, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Materials and Instruments
[0042] 1.1 Materials
[0043] E. coli strain Escherichia coli DH5α, BL21(DE3) was provided by the Cancer Research Institute of the Second Affiliated Hospital of Zhejiang University, the prokaryotic expression plasmid PET28a was purchased from Novagen, Germany, and the RNA extract (Trizol) was purchased from Gibcol BRL, USA. Ethyl ester, Oligo(dT) 15 , UNIQ-10 Column Genomic DNA Extraction Kit were purchased from Shanghai Sangon Bioengineering Company, M-MLV Reverse Transcriptase, RNasin, dNTPs, Taq DNA polymerase, agarose, T 4 DNA ligase, restriction endonucleases Nhe I, BamH I, HindIII, and Xba I were all purchased from Promega Company in the United States, and lymphocyte extract was purchased from Shanghai Hengxin Chemical Reagent Co., Ltd., GeneRuler TM DNA Ladder Mix was purchased from Fermentas Company in Germany, DNA Marker DL2000, Isopropyl-beta-D-thiogalactopyranoside (IPTG) was purchased from TaKaRa Company in Japan, SDS polya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com