Pharmaceutical Formulations and Methods of Making the Same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Testing of Various Formulations
[0048]This example demonstrates the effects of pH and buffer on etanercept at 50 mg / mL, and assesses the stability of a high concentration (100 mg / mL) solution without added phosphate buffer. The following formulations were tested.
TABLE 1Formulations for pH ScreenProteinPolysorbateFinalConcFormulation NameBufferExcipientsAddedpH(mg / mL)A45SuT10 mM9% sucrose0.004%4.550sodiumPS20acetateA52SuT10 mM9% sucrose0.004%5.250sodiumPS20acetateA58SuT10 mM9% sucrose0.004%5.850sodiumPS20acetate50_SAST_100NaClNone25 mM L-0.004%6.350arginine, 100 mMPS20NaCl, 1% sucrose100_SAST_100NaClNone25 mM L-0.004%6.3100arginine, 100 mMPS20NaCl, 1% sucrosePASST + BeOH25 mM25 mM L-0.004%6.350phosphatearginine, 100 mMPS20NaCl, 1% sucrose,0.9% benzyl alcoholPASST (control)25 mM25 mM L-0.004%6.350phosphatearginine, 100 mMPS20NaCl, 1% sucrose
[0049]Materials:
[0050]Enbrel drug substance in PASS (25 mM phosphate buffer, 25 mM L-arginine, 100 mM NaCl, 1% sucrose) at 50 mg / mL was used for th...
example 2
y
[0057]This study was a single-center, randomized, single-blind, crossover design in which 48 healthy men and women received single SC injections of 6 solutions.
[0058]Test formulations (detailed below in Table 9) were administered by a trained healthcare professional in 6 unique sequences with 8 subjects randomized to each sequence. Injections were administered in each quadrant of the anterior abdominal wall and administered approximately 1 hour apart. Within 30 seconds after each injection, subjects assessed their level of injection pain using a 100 mm Visual Analog Scale (VAS). Adverse events were collected from the beginning of the first injection through 30 days after the first injection. Safety follow-up phone calls were conducted on day 2 (24 hours after the sixth injection) and day 31 (+2 days).
TABLE 9Tested FormulationsSolutionDescriptionVolumeCompositionA.Negative pain1.010 mM sodium acetate, 9% (w / v)controlsucrose, 0.004% (w / v) polysorbate20, pH 5.2B.Commercial.09825 mM so...
example 3
Stability Testing of Formulation Candidates
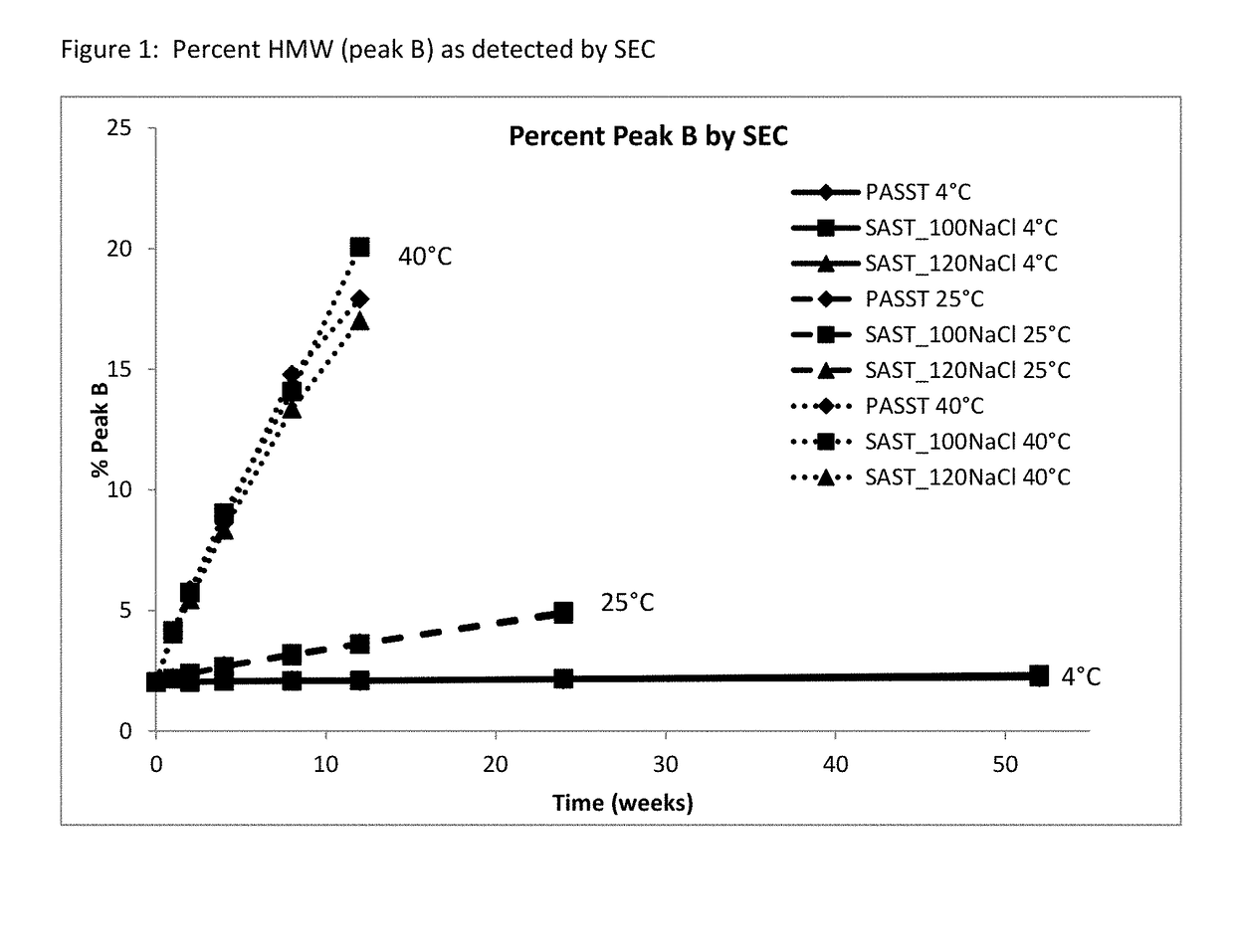
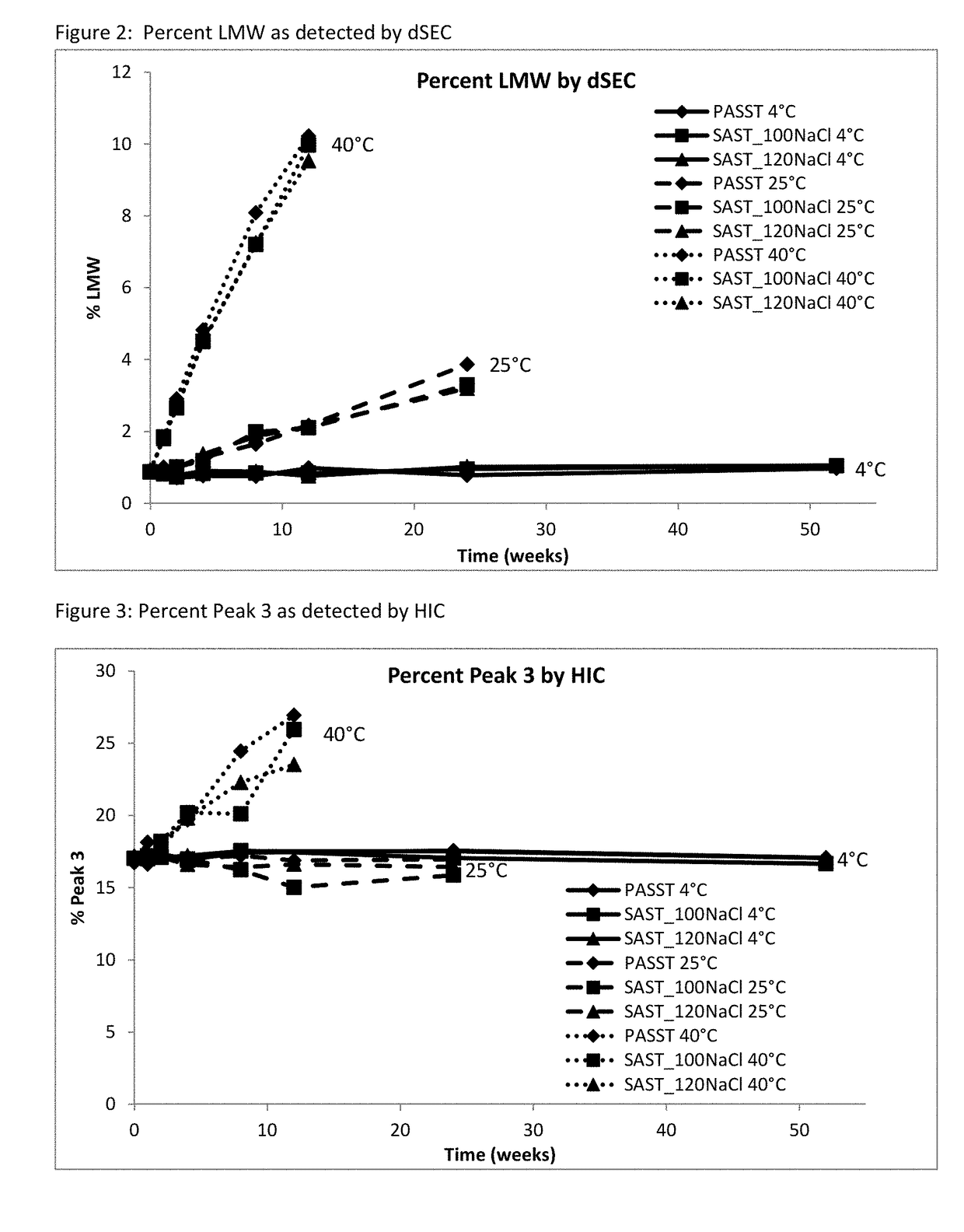
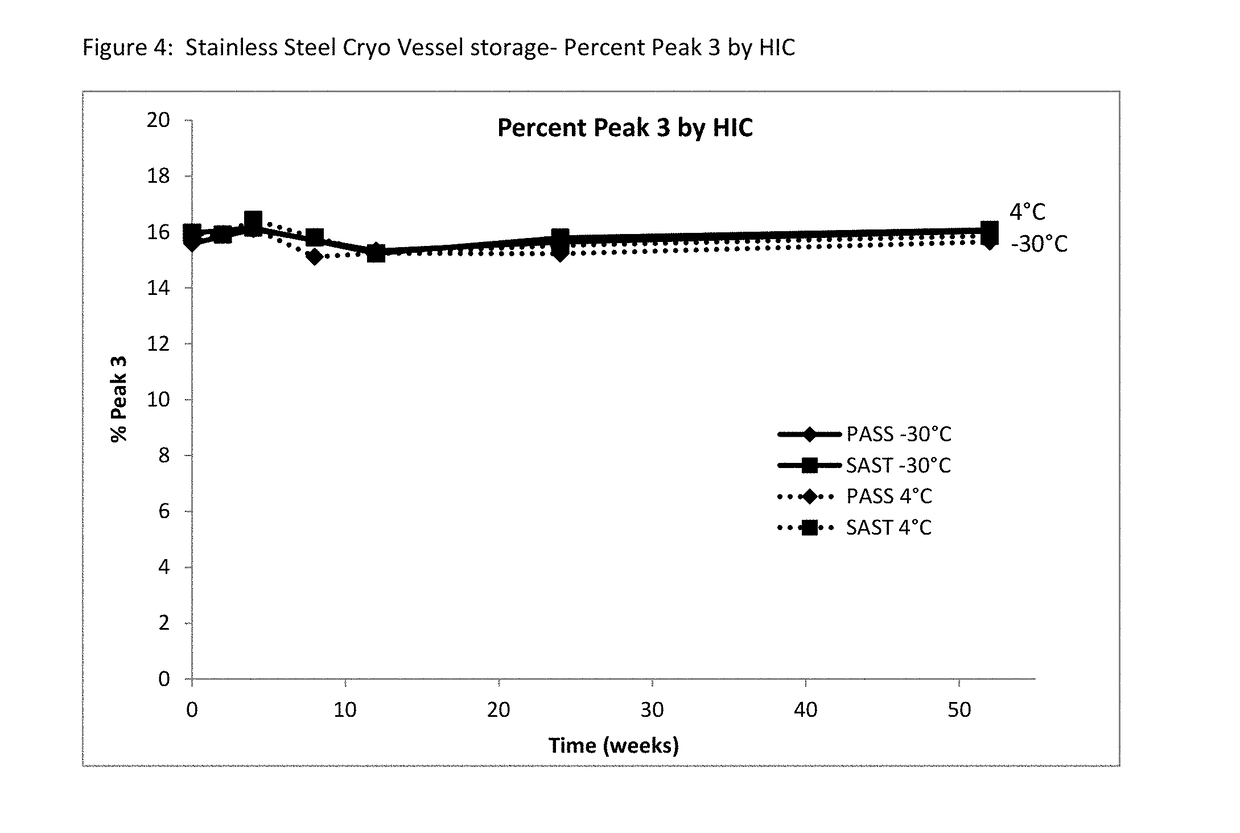
[0065]A long-term study was performed to monitor etanercept stability in several new formulation candidates at 50 mg / mL. The stability was assessed on 1 mL fills in 1 mL staked glass needle syringes using SE-HPLC, HIC HPLC, dSEC HPLC, and particulate matter (HIAC) after storage at 4° C., 25° C. and 40° C. Osmolality and protein concentration were tested at time zero only, and pH was tested at time zero and after 12 weeks of storage to confirm that there was no pH drift. The results of the study showed that the formulations tested remained similar to the current commercial formulation after 12 weeks at the accelerated temperature of 40° C., as well as 24 weeks at the recommended storage of 2-8° C. and at the accelerated temperature of 25° C.
TABLE 11Formulation conditions at 50 mg / mL etanerceptFormulationOtherNameBufferExcipientspHPASST25 mM25 mM L-arginine, 100 mM NaCl,6.3(control)phosphate1% sucrose, 0.010% polysorbate 20SAST_100NaClnone no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com