Kit for quantum dot nucleic acid detection of urinary tract infection-causing pathogens
A urinary tract infection and quantum dot technology, applied in the field of biomedicine, can solve the problems of complex preparation process, unfavorable promotion, complicated and cumbersome detection process, etc., and achieve high specificity, high throughput, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Preparation and use of the kit for quantum dot nucleic acid detection of urinary tract infection pathogens according to the present invention
[0114] 1. Quantum dot nucleic acid detection principle:
[0115] Molecularly hybridize the nucleic acid amplification product labeled with biotin with the probe on the detection membrane strip, and then combine biotin with quantum dots coupled with streptavidin, and observe each position of the detection membrane strip through a fluorescence detection instrument. Whether the probe hybridizes with the nucleic acid product is determined by spotting the light signal, so as to determine whether the sample contains the relevant target nucleic acid.
[0116] The capture probe is the 3' or 5' end of the oligonucleotide single-stranded DNA labeled with an amino group, and there is an intermediate arm between the amino group and the oligonucleotide single-stranded DNA, and the intermediate arm is a fatty acid C(n) chain or oligo dT (n) ...
Embodiment 2
[0237] Validation analysis of the present invention's kit for quantum dot nucleic acid detection of urinary tract infection pathogens
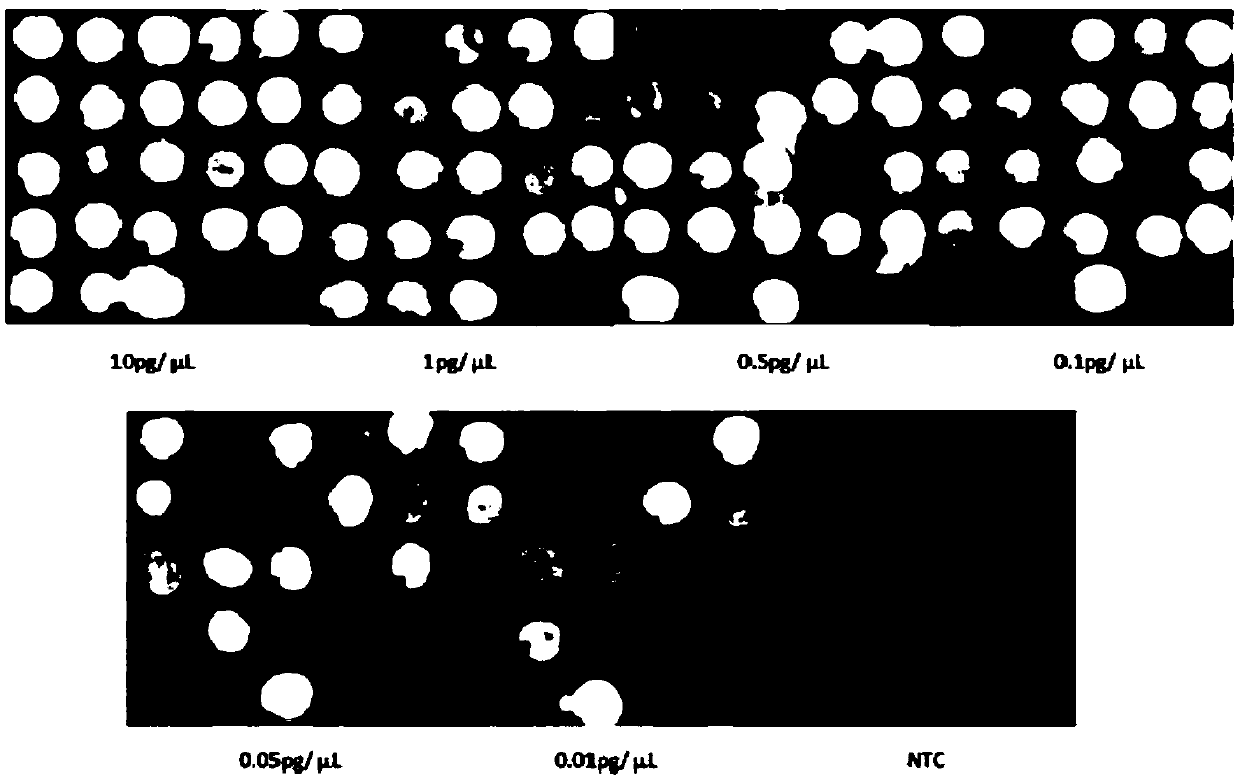
[0238] 1. Sensitivity detection
[0239] The genomic DNA of each pathogenic bacteria at the calibration concentration was serially diluted (10pg / μL, 1pg / μL, 0.5pg / μL, 0.1pg / μL, 0.05pg / μL, 0.01pg / μL), and passed the PCR-quantum dot fluorescence detection method The above concentrations of DNA were tested separately, and those with obvious bright spots were judged as positive results, and those without spots were judged as negative results. The detection is carried out according to the kit usage procedure in Implementation 1. For test results, see figure 1 .
[0240] 2. Specific detection
[0241] The reaction system was prepared according to the above-mentioned Example 1, and divided into 21 ul, and 4 ul of genomic DNA was added to each reaction system. The detected genomic DNA was Streptococcus pyogenes, Streptococcus agalactiae, Streptoco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com