Transmucosal administration of agents
A technology for mucosal drug administration and medicine, which is applied in the direction of antipyretics, anesthetics, anti-inflammatory agents, etc., can solve the problems related to the mucosal absorption of podocalyxin, and achieve the goal of improving absorption efficiency, convenience or effectiveness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0048] The present invention will be specifically described below, but the present invention is not limited by them.
reference example 1
[0049] Reference Example 1 Confirmation of the expression of podocalyxin in M-like cells in vitro
[0050] For example in literature (Kai H, Motomura Y, Saito S, Hashimoto K, Tatefuji T, Takamune N, Misumi S. Royal jelly enhances antigen-specific mucosal IgAresponse. Food SciNutr. 2013 Mar 6; 1(3): 222-227.) As described, 3×10 cells were inoculated on the membrane of a permeable cell culture chamber (Transwell) (Corning, pore size: 3 μm, 24 holes). 5 Caco-2 cells were left to stand overnight, and then the membrane of the permeable cell culture chamber was immersed in Eagle's MEM (20% FBS) containing 20% fetal bovine serum and 0.1 mM non-essential amino acids. , 0.1 mM NEAAEMEM) in a 24-well plate. The permeable cell culture chamber was moved to a 24-well plate added with new 20% FBS and 0.1 mM NEAAEMEM approximately every 3 days and cultured for 21 days to form a Caco-2 monolayer. After 21 days of culture, add 1 × 10 to the upper chamber of the permeable cell culture chamb...
reference example 2
[0053] Reference Example 2 Confirmation of the expression of podocalyxin in intestinal M cells of cynomolgus monkeys
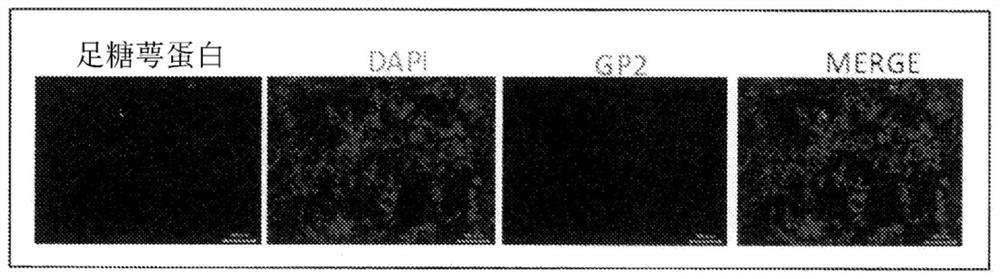
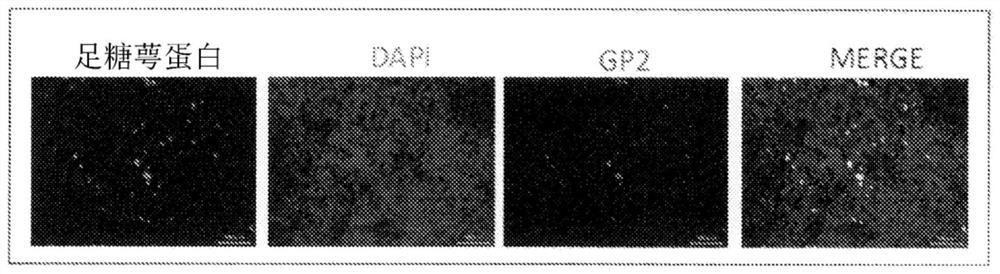
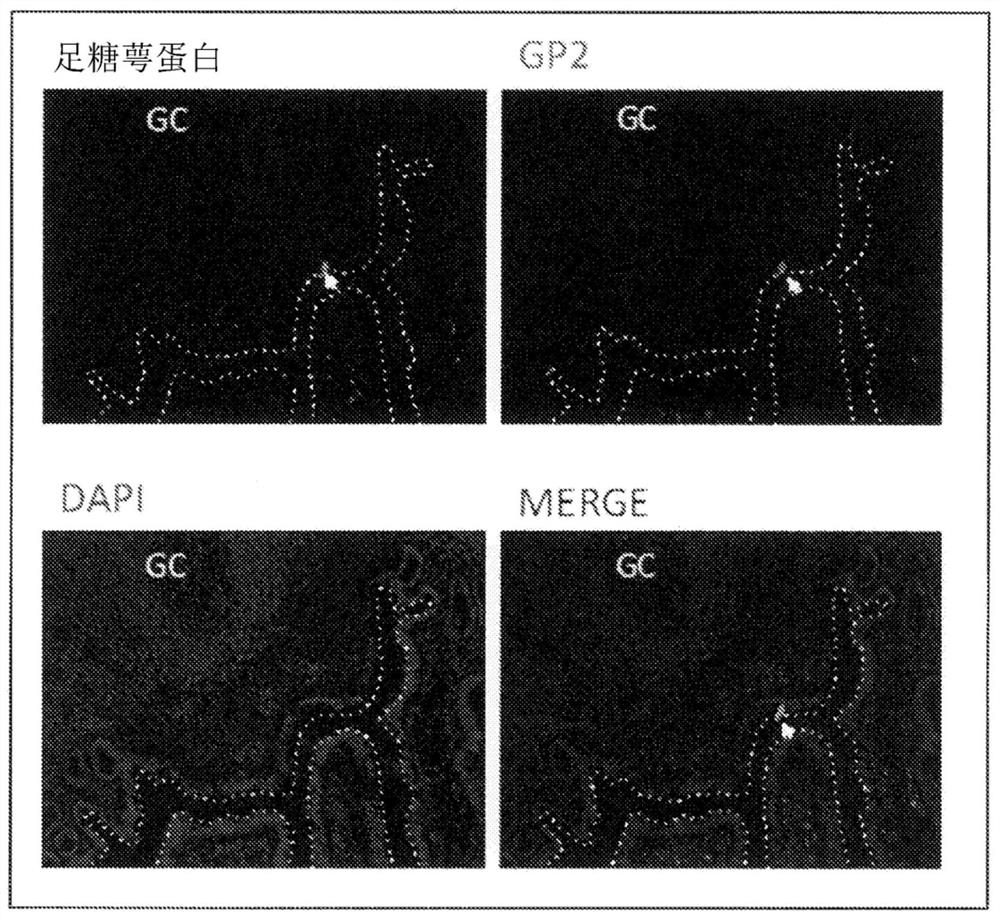
[0054] A portion of the cynomolgus monkey from the ileum side 30 cm from the cecum to the cecum was excised, embedded with an OCT embedding medium (Sakura Finetek), and frozen sections were prepared. The frozen sections containing Peyer's patches were fixed by immersion in cold acetone, and then masked by immersion in D-PBS containing 5% skim milk for 3 hours. After masking, use skim milk in D-PBS containing anti-podocalyxin antibody (R&D Systems), Alexa488-labeled donkey anti-goat IgG antibody, Alexa555-labeled anti-GP2 antibody, and diamino-phenyliodide (DAPI) To stain. The stained tissue sections were observed with a laser microscope (Keyence).
[0055] figure 2 3 shows an image observed by a laser microscope, and the expression of GP2, which is an M cell marker, was confirmed in the portion indicated by the arrow in the figure, and the expression of po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com