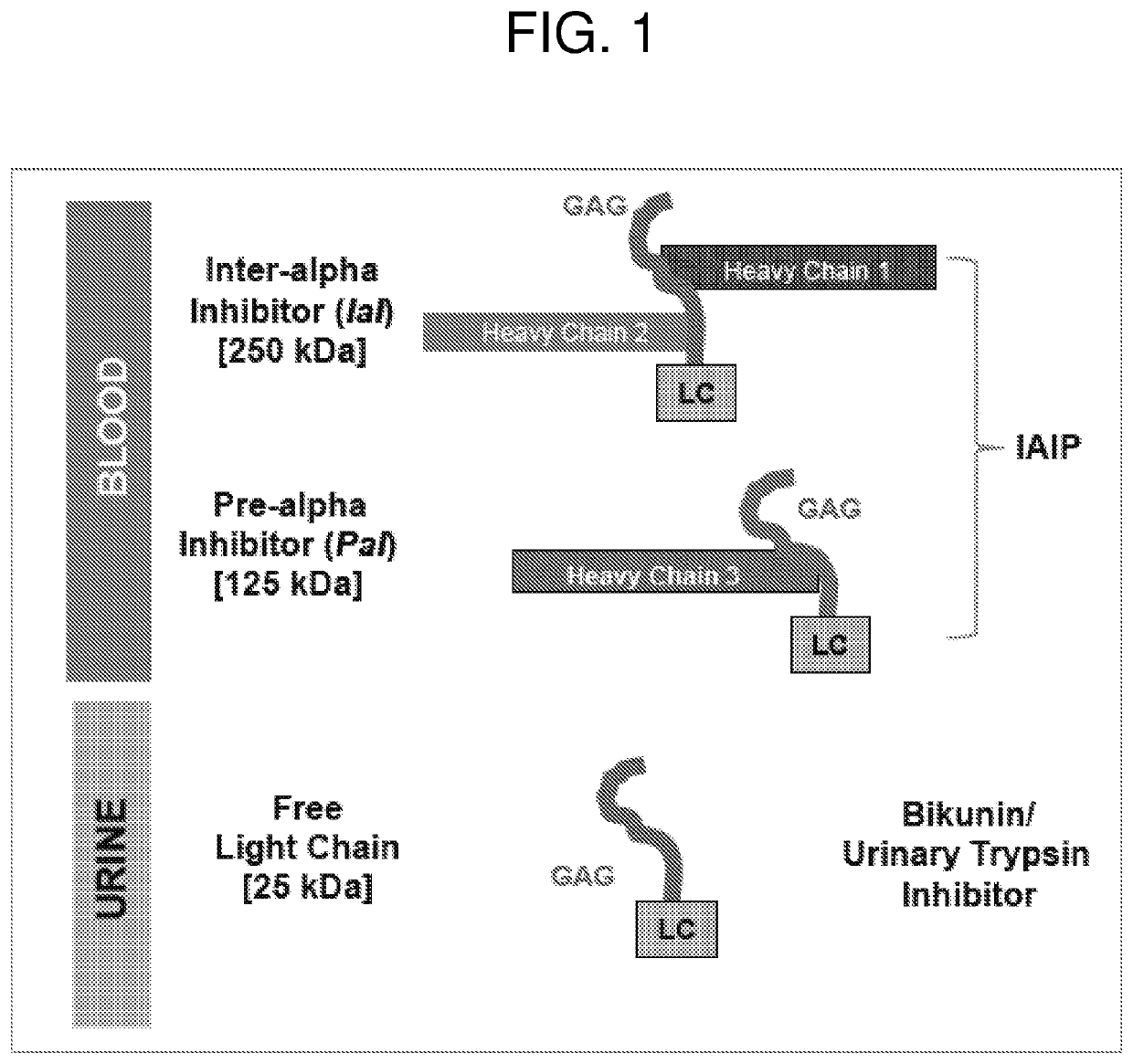
Methods for quantifying inter-alpha inhibitor proteins
a technology of interalpha inhibitors and proteins, applied in the field of methods for quantifying interalpha inhibitor proteins, can solve the problems of limitations of competitive iaip immunoassays in the assessment of active iaip in patient samples, and achieve the effect of reducing or ameliorating disorders and/or symptoms associated with them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
AIP Assay
[0203]Preparation of Biotinylated Heparin
[0204]Heparin (Heparin Sodium Injection USP, Sagent Pharmaceuticals, Cat # NDC 25021-400-30) was conjugated with biotin using the Biotin Hydrazide reagent (ApExBIO, Cat # A87007) according to the manufacturer's instructions. Briefly, 1000 IU heparin solution was mixed with 0.25 mg crosslinker reagent EDC (1-(3-Dimethylaminopropyl)-3-3ethylcarbodiimide hydrochloride, Alfa Aesar Cat # A10807) and 0.5 mM Biotin hydrazide that had been previously dissolved in DMSO in 0.1 M MES buffer pH 4.7 with gentle mixing at room temperature for 3 hrs. The unconjugated biotin and buffer exchange was carried out by ultrafiltration on an Amicon Ultra centrifugal filter device with 5 kDa cut off filter membrane (Millipore). Following dilution in d-H2O, the biotinylated Heparin was ready for use in the assay.
[0205]“Sandwich Type” Heparin-IAIP ELISA
[0206]Purified mouse monoclonal antibody against the light chain of human IAIP (MAb 69.26) was immobilized o...
example 2
[0207]Preparation of Biotinylated Endotoxin / LPS (Lipopolysaccharide):
[0208]Lipopolysaccharide (LPS / endotoxin) from Escherichia coli 055:B5 (Sigma Catalog # L2280) was labeled with biotin using Biotin Hydrazide reagent (ApExBIO, Cat # A87007) according to the manufacturer's instructions and similarly to the protocol used for heparin. 10 mg LPS was reconstituted in 0.1 M MES buffer and 2.5 mM Biotin-Hydrazide and 2.5 mg EDC (1-(3-Dimethylaminopropyl)-3-3ethylcarbodiimide hydrochloride, Alfa Aesar Cat # A10807) were gently mixed for 3 hrs at room temperature. The removal of unconjugated LPS and buffer exchange were carried out by ultrafiltration on Amicon Ultra centrifugal filter device with a 5 kDa cut off filter membrane (Millipore). Following dilution in d-H2O, the biotinylated LPS was ready for use in the assay.
[0209]“Sandwich Type” LPS-IAIP ELISA
[0210]Similar to the heparin-IAIP protocol described above, purified mouse monoclonal antibody against the light chain of human IAIP...
example 3
of Blood Samples from Patients Diagnosed with Severe Community Acquired Pneumonia (sCAP)
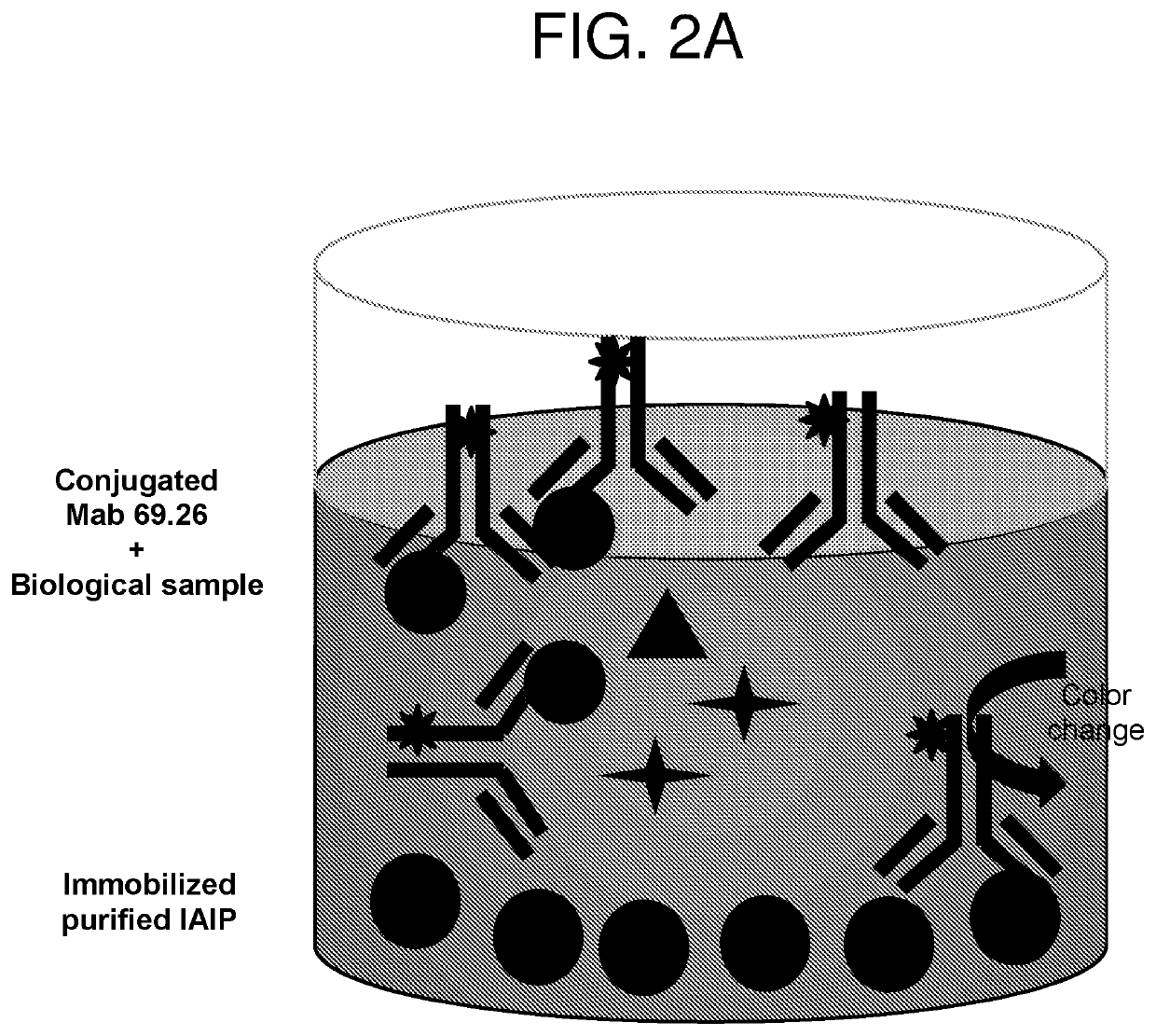
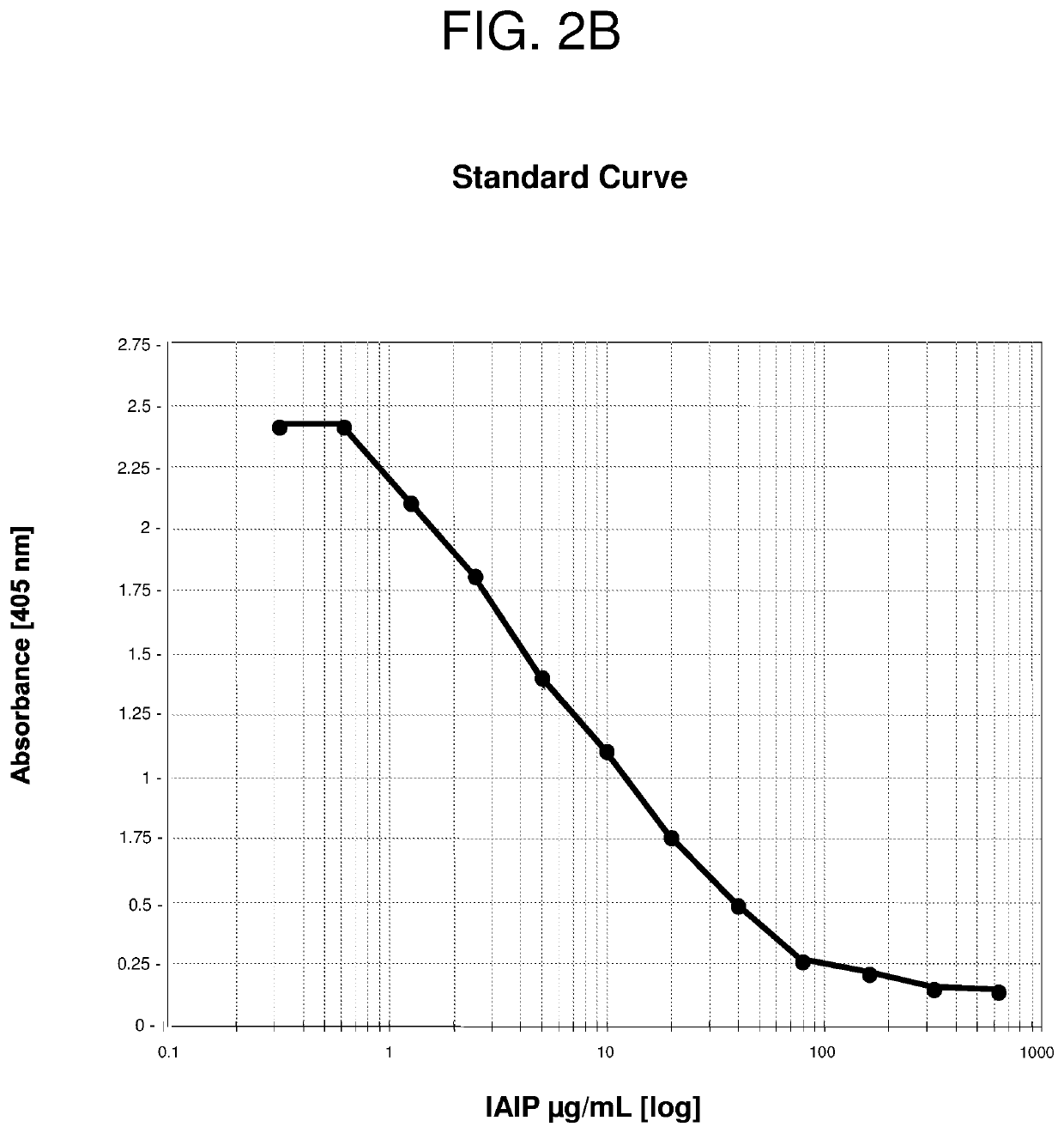
[0211]Serial blood samples were collected from patients with a confirmed diagnosis of sCAP who were hospitalized in the Intensive Care Unit at Rhode Island Hospital. 16 patients were enrolled in the study and plasma was collected on days 0 (time of admission to the ICU), 1, 3 and 7. The level of IAIP was determined using the established competitive ELISA (FIG. 2A) and both sandwich-type ELISAs using biotinylated heparin or biotinylated LPS as detecting molecules (FIG. 3A). Blood samples from 95 healthy controls aged between 17 to 71 years old (obtained from healthy blood donors and purchased from Rhode Island Blood Center) were included in this study to compare IAIP levels in health controls to the levels measured in sCAP patients. The results are shown in FIGS. 4, 5, and 6.
[0212]The results indicate that IAIP levels were significantly lower in sCAP patients compared to healthy controls at the ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com