Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
a technology of diphenylprop-2-en-1 and derivatives, which is applied in the field of liver disorders using 1, 3diphenylprop-2-en-1 derivatives, can solve the problems of inability to accurately diagnose nafld or nash, inability to do analysis for every single study, and insufficient histological features to distinguish properly nafld or nash
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effects of Compounds of General Formula (I) on Liver-Specific Biochemical Indexes
Materials & Methods
[0194]1-[4-methylthiophenyl]-3-[3,5-dimethyl-4-carboxydimethylmethyloxyphenyl]prop-2-en-1-one (Cpd 29 of WO2004 / 005233) has been formulated as hard shell capsules containing 5, 10 or 20 mg of the compound. The compound (80 mg) was administered orally once daily for 28 days. The study has been performed in two parallel groups in double-blind conditions: placebo or Cpd 29 of WO2004 / 005233.
[0195]The tolerability and safety of once-a-day administrations, as well as the efficacy in improving plasma lipids and glucose homeostasis compared with placebo, were evaluated in two pilot trials using relevant biochemical parameters. The data were used to calculate the percentage of change due to the compound when compared to the placebo after 28 days of treatment.
[0196]Results & Conclusions:
[0197]A first pilot, double-blind, placebo controlled, randomized study has been performed in patients suffer...
example 2
Animal Models for Testing Liver-Specific Properties of Compounds of General Formula (I)
Materials & Methods
[0202]Animal Model and Treatment: ob / ob Mice
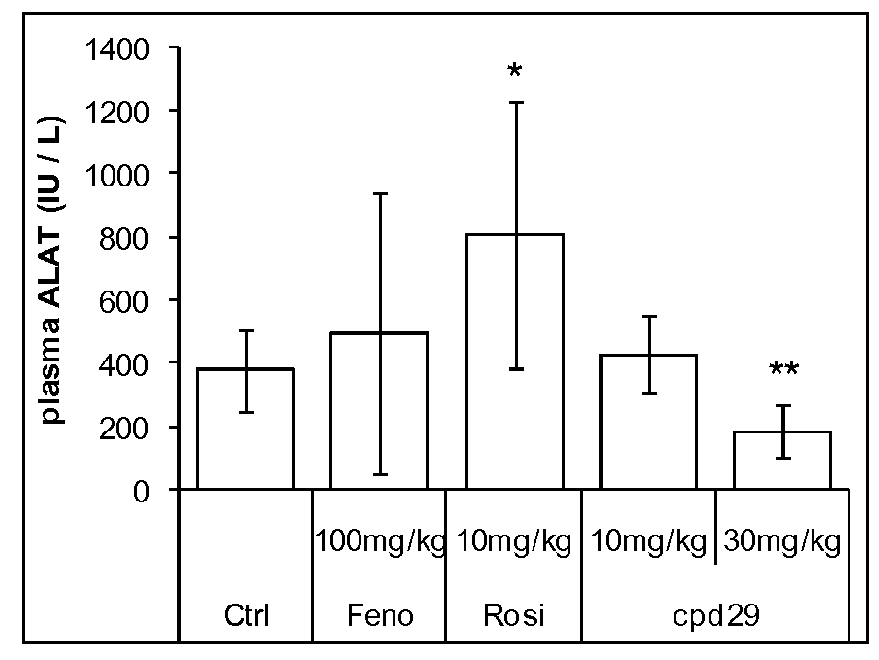
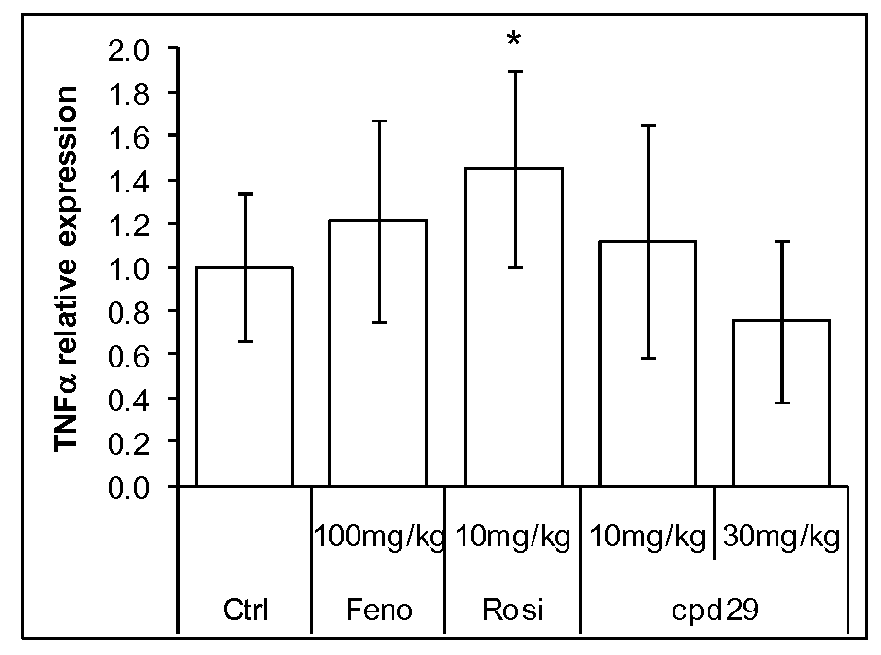
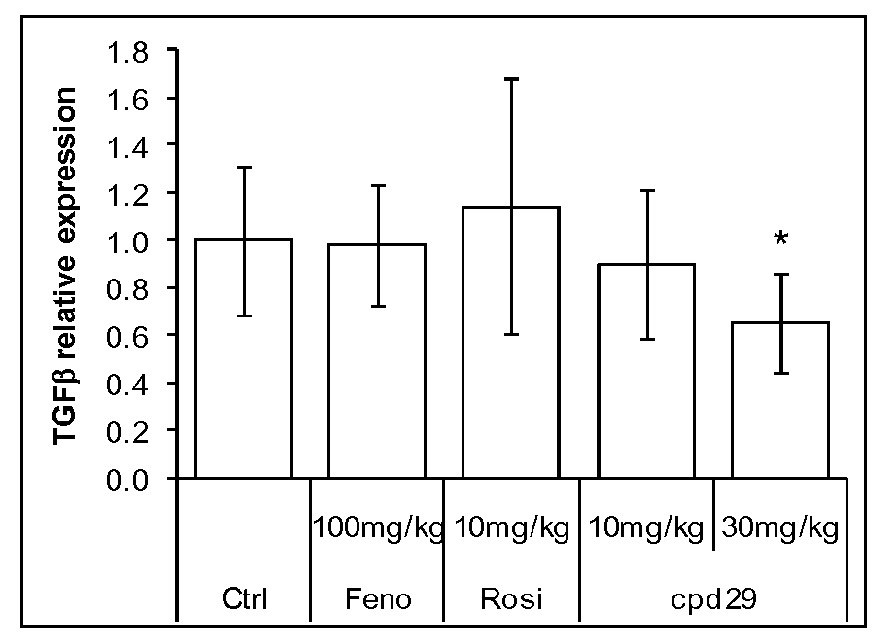
[0203]Male ob / ob mice (8 weeks of age) were purchased from Charles River (L'Arbresle, France) and were kept on a 12-hour light / dark cycle at a constant temperature of 20±3° C. After a 1 week acclimation, mice were separated in groups of 8 animals selected such that the distribution of their body weight and their 6 hours fasting glycemia determined before the experiment were uniform. Animals were fed a standard chow diet (R03, SAFE) and treated during 26 days with the compounds of interest. Compounds, including the Compound 29 of WO2004005233 (Cpd 29, at 10 or 30 mg / kg / day), Fenofibrate (100 mg / kg / day) and Rosiglitazone (10 mg / kg / day) were administered daily by gavage. Control animals were treated with vehicle only (Carboxymethlycellulose 1%+Tween-80 0.1%). Animals had access to food and water ad libitum.
Animal Model and Treatment: Stud...
example 3
In Vitro / Ex Vivo Models for Testing Liver-Specific Properties of Compounds of General Formula (I)
[0228]Some in vitro / ex vivo models have been established for screening compounds that can have a positive effect on liver fibrosis, oxidative stress, and / or liver inflammation. In fact, a key event in liver fibrosis is the activation of hepatic stellate cells (HSC). After hepatocyte damage, this cell type becomes activated and starts to proliferate (Sato M et al., 2003). Activated HSCs (for example, rat or human HSC isolated from livers or rat HSC-T6 cell line) can be activated and produce excessive amounts of extracellular matrix compounds and inhibitors of matrix degradation. This approach has been used to show the positive effect of compounds such as curcumin (Xu et al., 2003), thiazolidinediones (Miyahara T et al., 2000), or 17beta-estradiol (Liu Q et al., 2004).
[0229]The in vitro / ex vivo models described above allow comparing liver-specific activities of compounds of General Formula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com