L-ornithine phenyl acetate and methods of making thereof
A technology of phenylacetate and ornithine, which is applied in the fields of medicinal chemistry, biochemistry and medicine, and can solve the problems of increased osmotic pressure, overload, difficult intravenous administration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
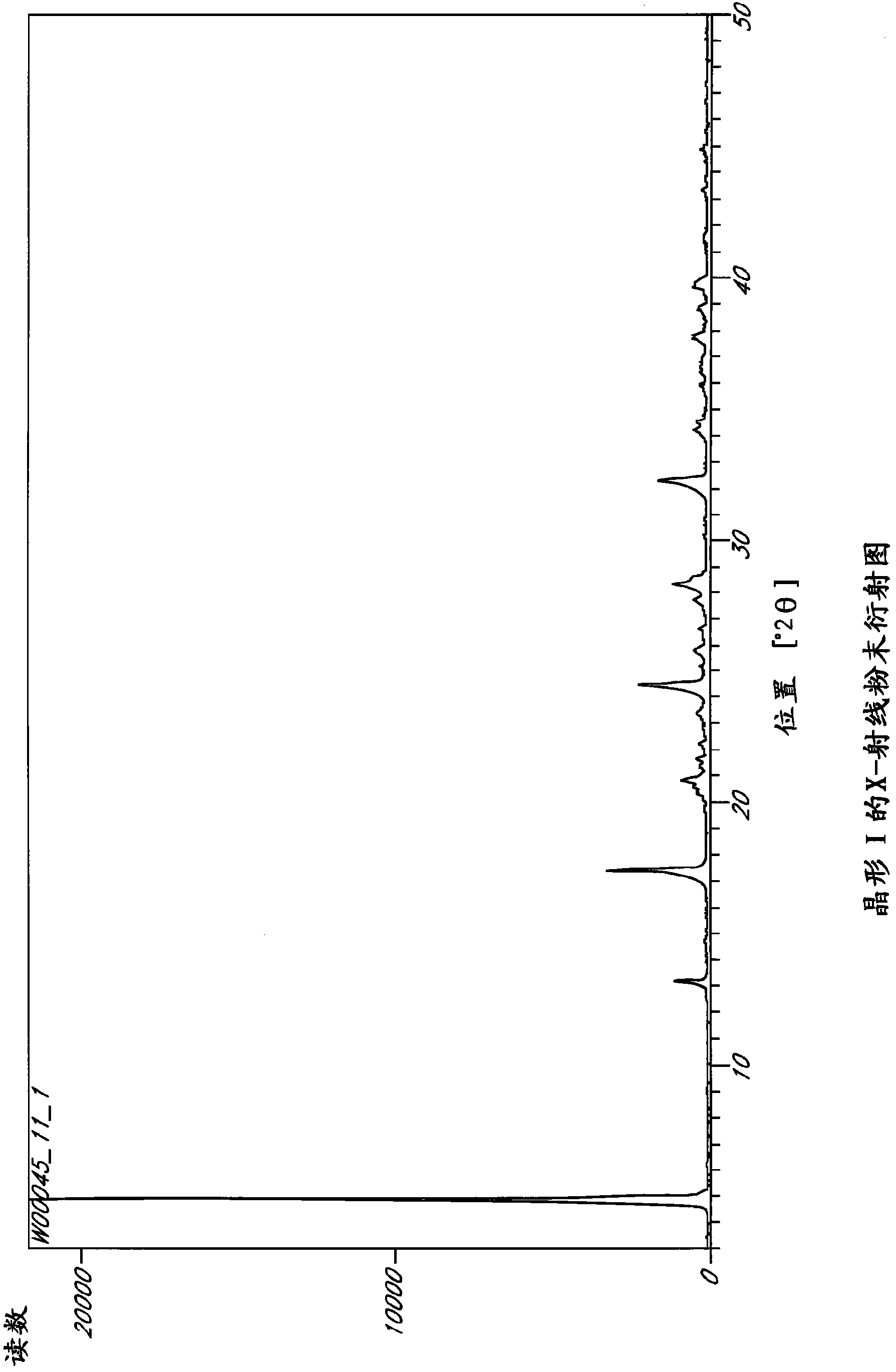
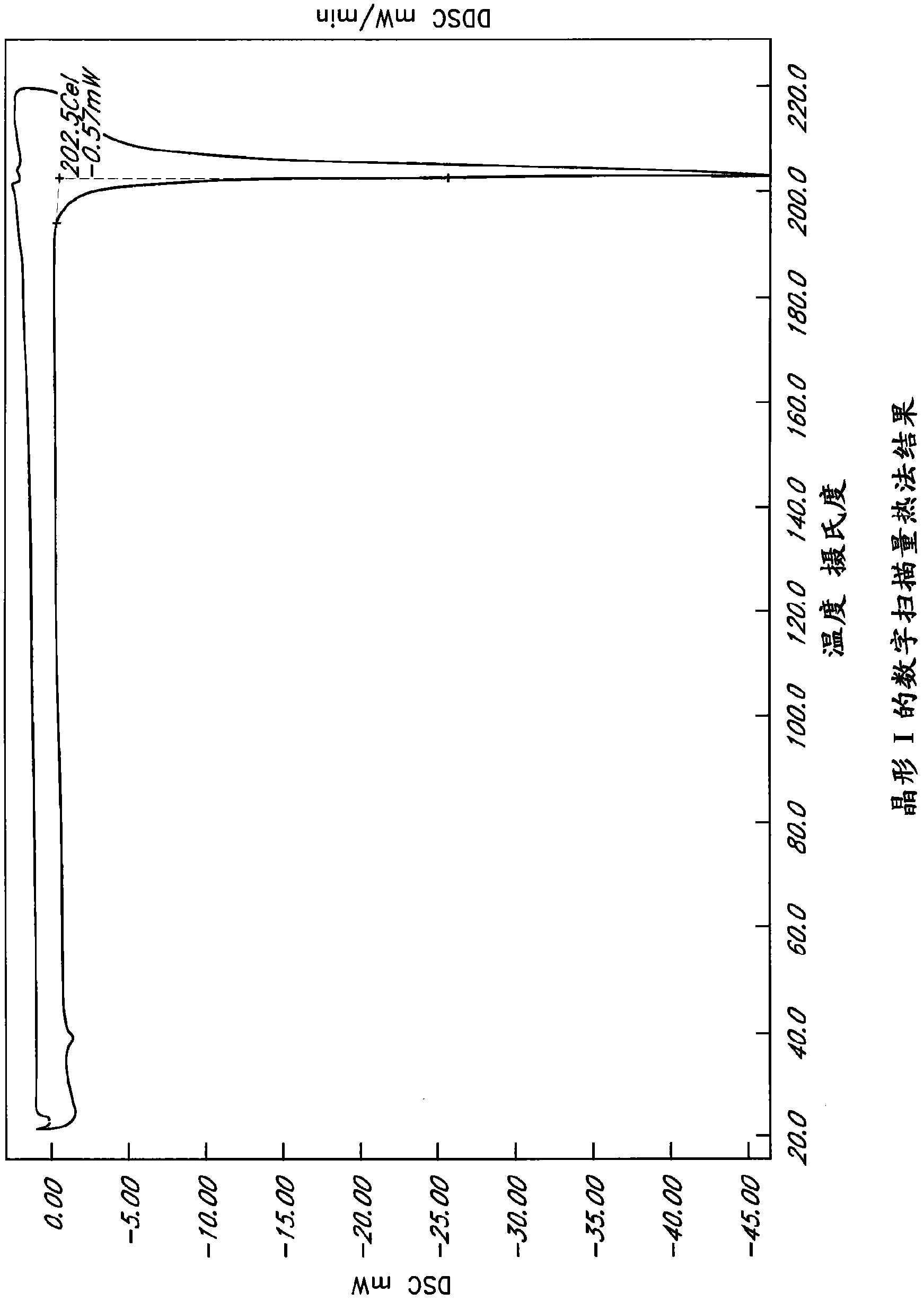
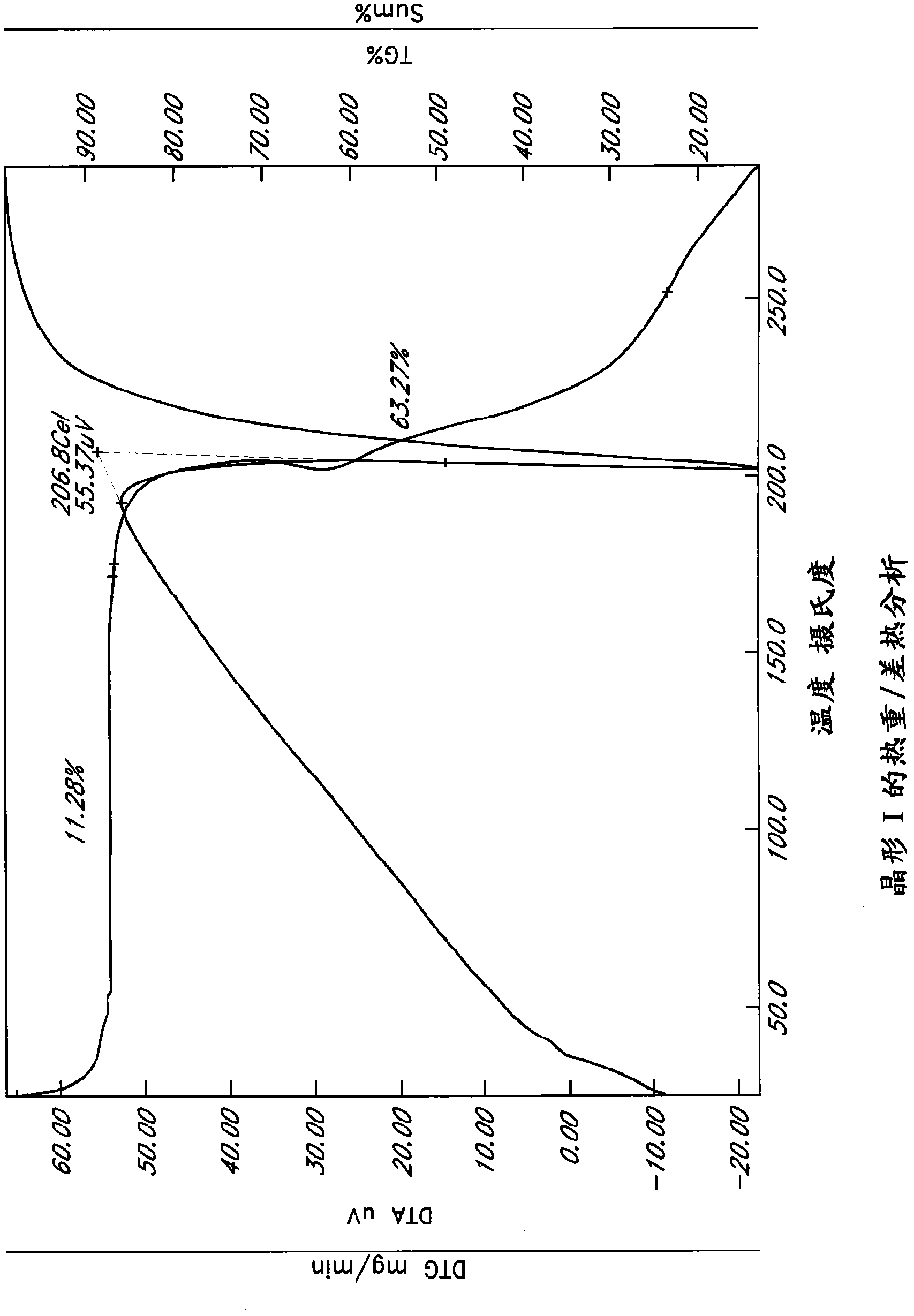
[0234] Example 1: Precipitated crystal form
[0235] Saturated solutions of L-ornithine phenylacetate were subjected to temperature cycling, flash cooling, evaporation or anti-solvent addition as described above. The precipitate was analyzed by PLM and XRPD to determine the crystalline form, if any. The results are summarized in Table 5.
[0236] Six unique crystal forms, Forms I-VI, were identified from precipitation studies. However, Forms IV and VI were obtained from acetic acid solution, and NMR results confirmed that these forms were L-ornithine acetate. Meanwhile, Test 540-611 utilized a sample of L-ornithine phenylacetate initially isolated by addition of ethanol back solution. Many of these examples yielded Form I, which is an ethanol solvate, so it can be assumed that these samples initially contained residual ethanol. Therefore, Form I may not reproduce under certain conditions if the original sample does not contain residual ethanol.
[0237] 5-Example of Pre...
Embodiment 2
[0261] Embodiment 2: characteristic dissolution study
[0262] Intrinsic dissolution rates of crystalline forms I, II and III were measured at pH 1.0, 4.5 and 6.7. The results are reproduced in Table 6 below. In each case, complete dissolution was achieved in less than 3 minutes. Surprisingly, a pH dependence was observed for Form II: the intrinsic dissolution rate increased with increasing pH. In contrast, the dissolution rates of forms I and III appear to be independent of pH.
[0263] Table 6 - Calculated Intrinsic Dissolution Rates (mg / cm 2 / s)
[0264] 1.0
Embodiment 3
[0265] Example 3: Solubility Studies
[0266] The solubility of L-ornithine phenylacetate was approximately estimated according to the method described above. 24 solvent systems were tested: 1,4-dioxane, 1-butanol, ethanol, acetone, benzonitrile, cyclohexane, DCM, DMSO, EtOAc, heptane, IPA, IPA (1% H 2 O), MeCN, MeCn (1%H 2 O), MEK, MeOAc, methanol, MIBK, nitromethane, THF, THF (1% H 2 O), toluene and water. L-ornithine phenylacetate showed solubility in water, but was substantially insoluble in the remaining solvent system.
[0267]A slurry of L-ornithine phenylacetate in water was also prepared and filtered. The filtrate concentration was analyzed by HPLC, and the result showed that the solubility of L-ornithine phenylacetate was about 1.072 mg / mL.
[0268] Solubility HPLC determinations were also performed for five solvents: ethanol, acetone, methanol, DMSO and IPA. These results are summarized in Table 7.
[0269] Table 7 - HPLC Solubility Determination
[0270] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com