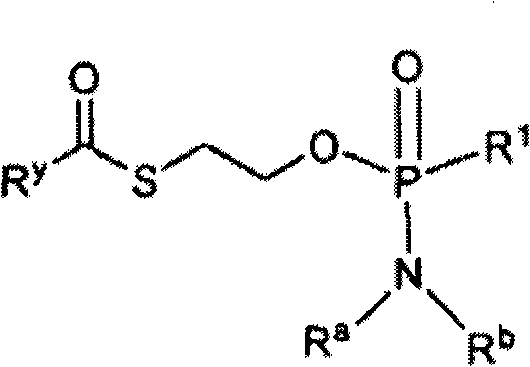
Compounds and pharmaceutical compositions for the treatment of viral infections
A technology of compounds and antiviral agents, applied in the direction of carbohydrate active ingredients, botanical equipment and methods, applications, etc., can solve the problem that vaccines cannot help patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0675] Preparation of A550(NM204), a hydroxy-tBuSATE N-benzyl phosphoramidate derivative of L-2′,3′-dideoxyadenosine L-ddA
[0676]
[0677] NM204, A550
[0678] Synthetic scheme
[0679]
[0680] Synthesis of Carboxylic Acid 2
[0681]
[0682] Methyl 2,2-dimethyl-3-hydroxypropionate (965 μL, 7.57 mmol) was added dropwise to 4,4′-dimethoxytrityl chloride (2.82 g, 8.33 mmol) at room temperature In a stirred solution in dry pyridine (7.6 mL). The reaction mixture rapidly turned into a red solution and then an orange suspension (approximately 30 minutes), and this suspension continued to stir overnight. Carefully pour the mixture over saturated NaHCO 3 Aqueous solution (30mL), with Et 2 O (3 x 20 mL) extracted the product. The combined organic extracts were washed with brine (20 mL), dried (Na 2 SO 4 ), and the volatiles were removed under reduced pressure. The resulting oil was co-evaporated with toluene and the residue was subjected to flash column chromatogr...
Embodiment 2
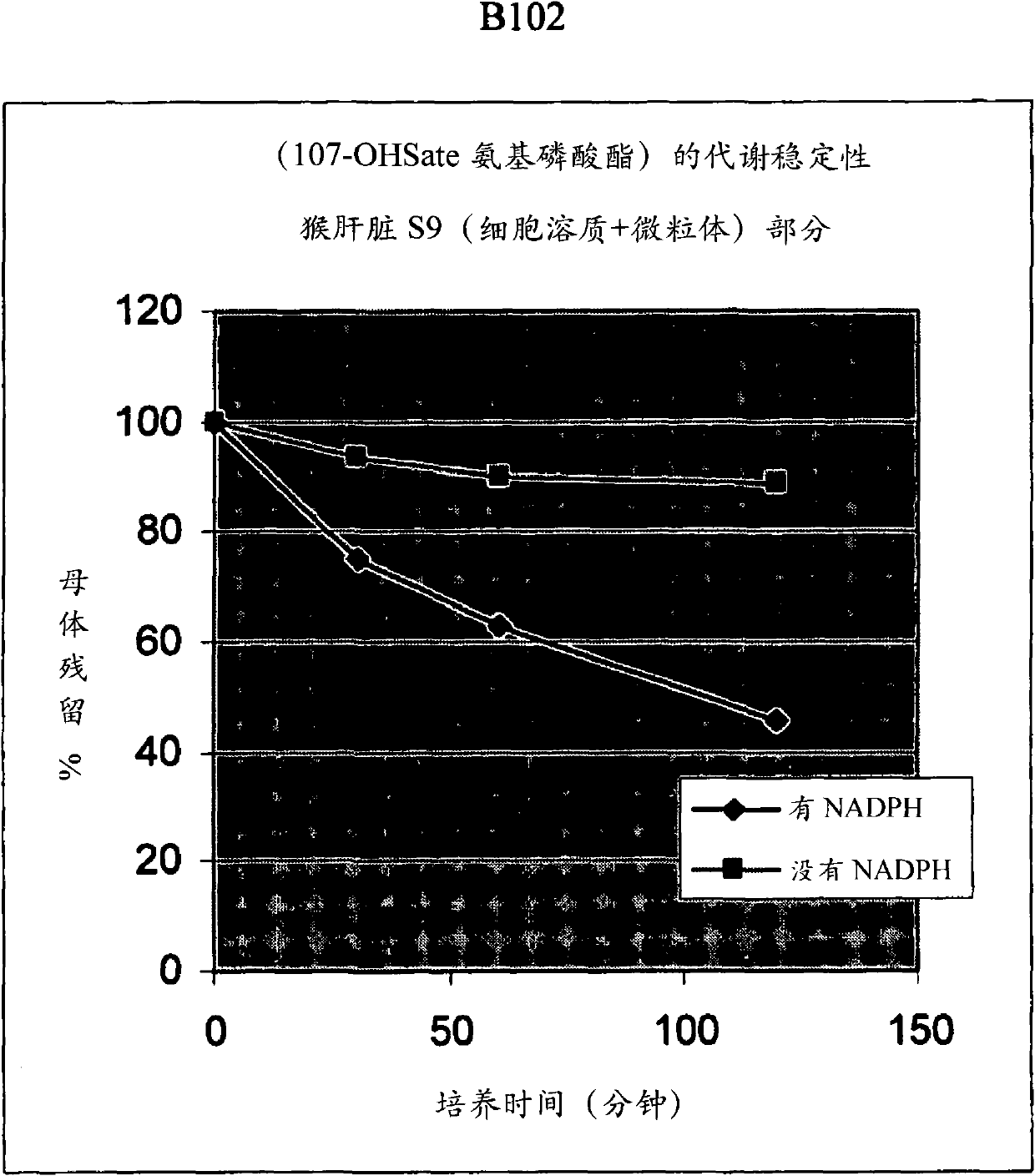
[0696] B102, Preparation of Hydroxy-tBuSATE N-Benzyl Phosphoramidate Derivatives of 2′-C-Methylcytidine
[0697]
[0698] Process A:
[0699] Synthesis of H-phosphonic acid monoester 5
[0700]
[0701] carboxylic acid 3 Synthesis:
[0702] To 2,2-dimethyl-3-hydroxypropionic acid methyl ester ( 1 , 15ml, 117.6mmol) to a stirred solution in a mixture of anhydrous dichloromethane (590ml) and triethylamine (23ml), triphenyldichloromethane (1.2 equivalents, 39.3g) and 4-dimethyl Aminopyridine (0.1 equiv, 1.44 g). The reaction mixture was refluxed overnight. Carefully pour the mixture over saturated NaHCO 3 On aqueous solution, the product was extracted with dichloromethane and washed with water. The combined organic extracts were evaporated under reduced pressure to give the crude compound 2 , the crude product was used in the next step without further purification. The resulting oil was dissolved in a mixture of dioxane (350ml) and aqueous NaOH (30%, 350ml). The...
Embodiment 3
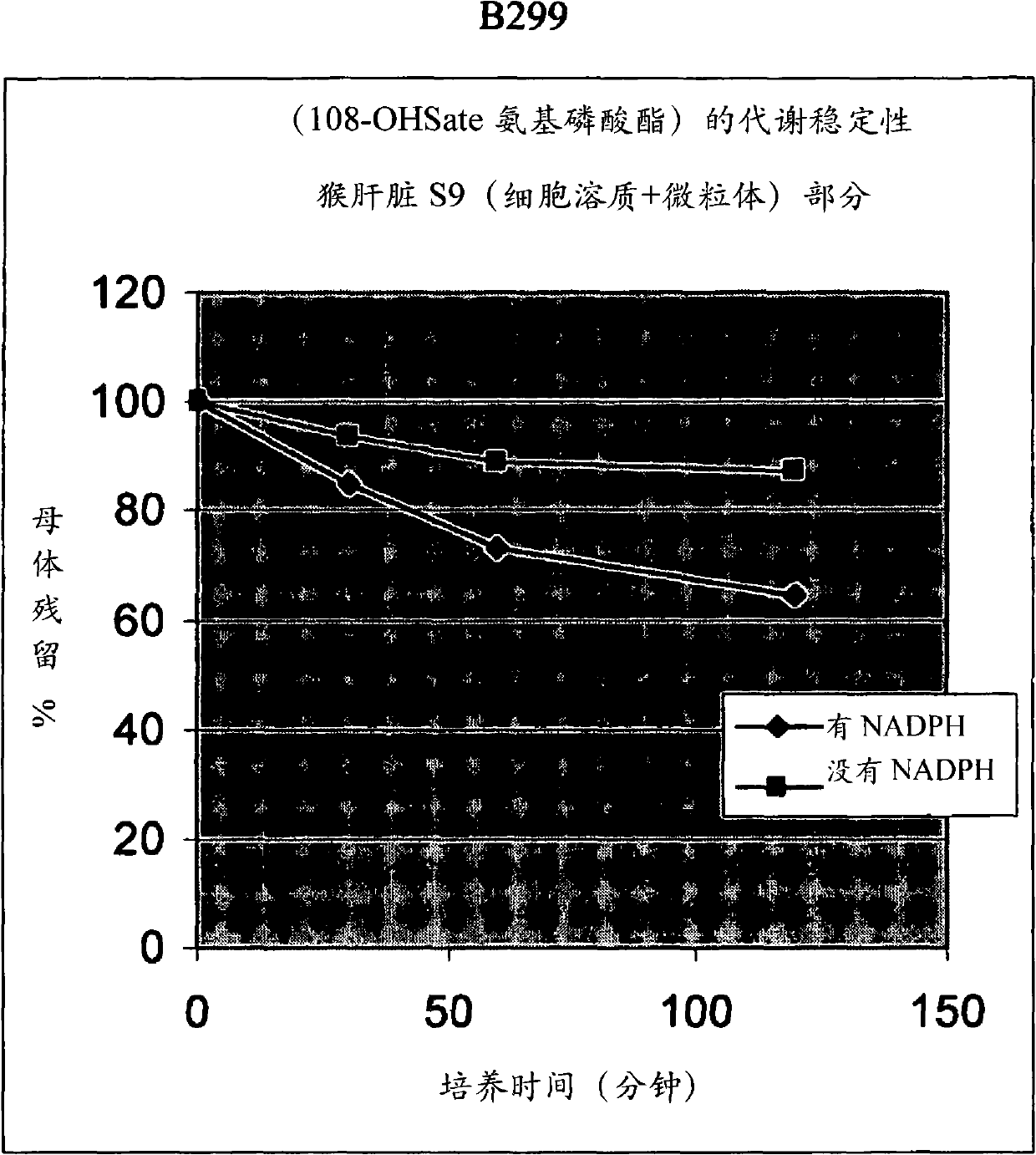
[0777] B299, Preparation of Hydroxy-tBuSATE N-Benzyl Phosphoramidate Derivatives of 2′-C-Methylguanosine
[0778]
[0779] Process A
[0780] Synthesis scheme:
[0781]
[0782] 2'-C-methylguanosine (NM108) (3g, 10.10mmol) and compound 5 [about 5 For the synthesis of , see Example 2] (6.48 g, 11.10 mmol) was co-evaporated with anhydrous pyridine and dissolved in this solvent (152 mL). Pivaloyl chloride (2.48 mL, 20.18 mmol) was added dropwise at -15 °C and the solution was stirred at the same temperature for 2 h. The reaction mixture was diluted with dichloromethane and washed with aqueous ammonium chloride (NH 4 Cl 0.5M) to neutralize. In dichloromethane / 0.5M NH 4 After extraction with aqueous Cl solution, the organic phases were combined and washed with Na 2 SO 4 Dry, evaporate under reduced pressure (bath temperature not exceeding 30°C) and co-evaporate twice with toluene. The crude mixture was purified by flash column chromatography on silica gel (eluent: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com