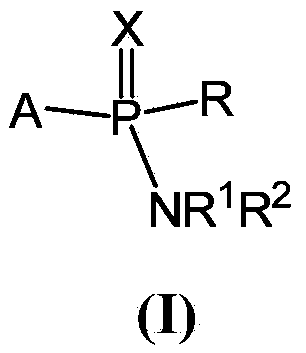
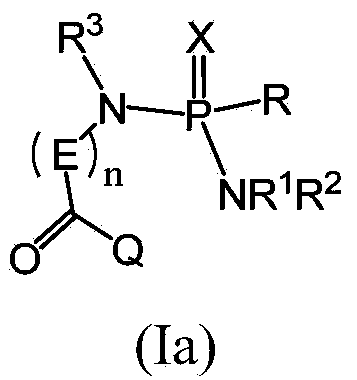
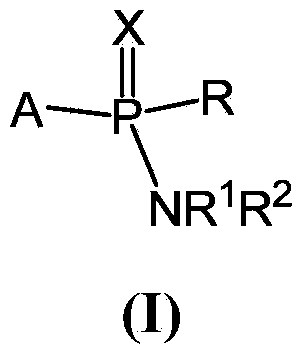
Compounds and pharmaceutical compositions for the treatment of viral infections
A technology of compounds and solvates, applied in the field of compounds and pharmaceutical compositions for the treatment of viral infections, can solve problems such as poor ability of hepatitis C virus to grow effectively
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0687] Preparation of compound 3
[0688]
[0689] Synthesis of Compound 1:
[0690]
[0691] To a stirred suspension of 2-amino-6-chloro-purine (1.8g, 1.2eq) in dry toluene (86ml) was added N,O-bis(trimethylsilyl)acetamide (8.4ml, 4eq ). The mixture was stirred at 120°C for 30 minutes (complete dissolution). 1,2,3,4-Tetra-O-benzoyl-2-C-methyl-D-ribofuranose (5 g, 1 equiv) and trimethylsilyltrifluoromethanesulfonic acid were then added at room temperature ester (3.9ml, 2.5eq) and the reaction mixture was stirred at 120°C for 3 hours. The mixture was then diluted in DCM and washed with saturated NaHCO 3 The solution was washed twice with NaCl solution and purified by silica gel chromatography (petroleum ether / EtOAc) to give compound 1 in 45% yield. MS (ESI, EI + )m / z=628(MH + ).
[0692] Synthesis of compound 2:
[0693]
[0694] To a stirred solution of compound 1 (17 g, 1 equiv) in MeOH (270 ml, 10 ml / mol) was added NaOCH at room temperature 3 (64g, 4.4 equiv...
Embodiment 2
[0699] Preparation of Compound 4
[0700]
[0701] Carbonyldiimidazole (10 mg, 2 equiv) was poured onto a solution of compound 3 (20 mg, 1 equiv) in anhydrous DMF (400 µl) at 0°C. The reaction mixture was stirred: 15 minutes at 0 °C and 2 hours at room temperature. Then by silica gel chromatography (C 18 , H 2 O / MeOH) to purify the crude material to give compound 4 as a white solid in 62% yield. MS (ESI, EI + )m / z=673(MH + ).
Embodiment 3
[0703] Preparation of compound 6
[0704]
[0705] Synthesis of compound 5:
[0706]
[0707] To a stirred solution of compound 1 (1 g, 1 equiv) in EtOH (13 ml) was added cyclopropylamine (1.1 ml, 10 equiv) at room temperature. The reaction mixture was refluxed for 16 hours, cooled to room temperature and concentrated under reduced pressure. The crude material was used as such in the next step.
[0708] The crude product from the previous step (1 eq) was dissolved in MeOH (32 ml). Then NaOMe (290 mg, 3.3 equiv) was added to the mixture at room temperature. The reaction mixture was stirred at room temperature for 2 h, neutralized to pH 7 with AcOH and chromatographed on silica gel (C 18 DCM / MeOH) to give compound 5 as a white solid in 93% yield. MS (ESI, EI + )m / z=337(MH + ).
[0709] Synthesis of Compound 6:
[0710]
[0711] Following the procedure as described for compound 3, compound 6 was prepared from compound 5 (300 mg, 1 equiv) and L-leucine ethyl este...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com