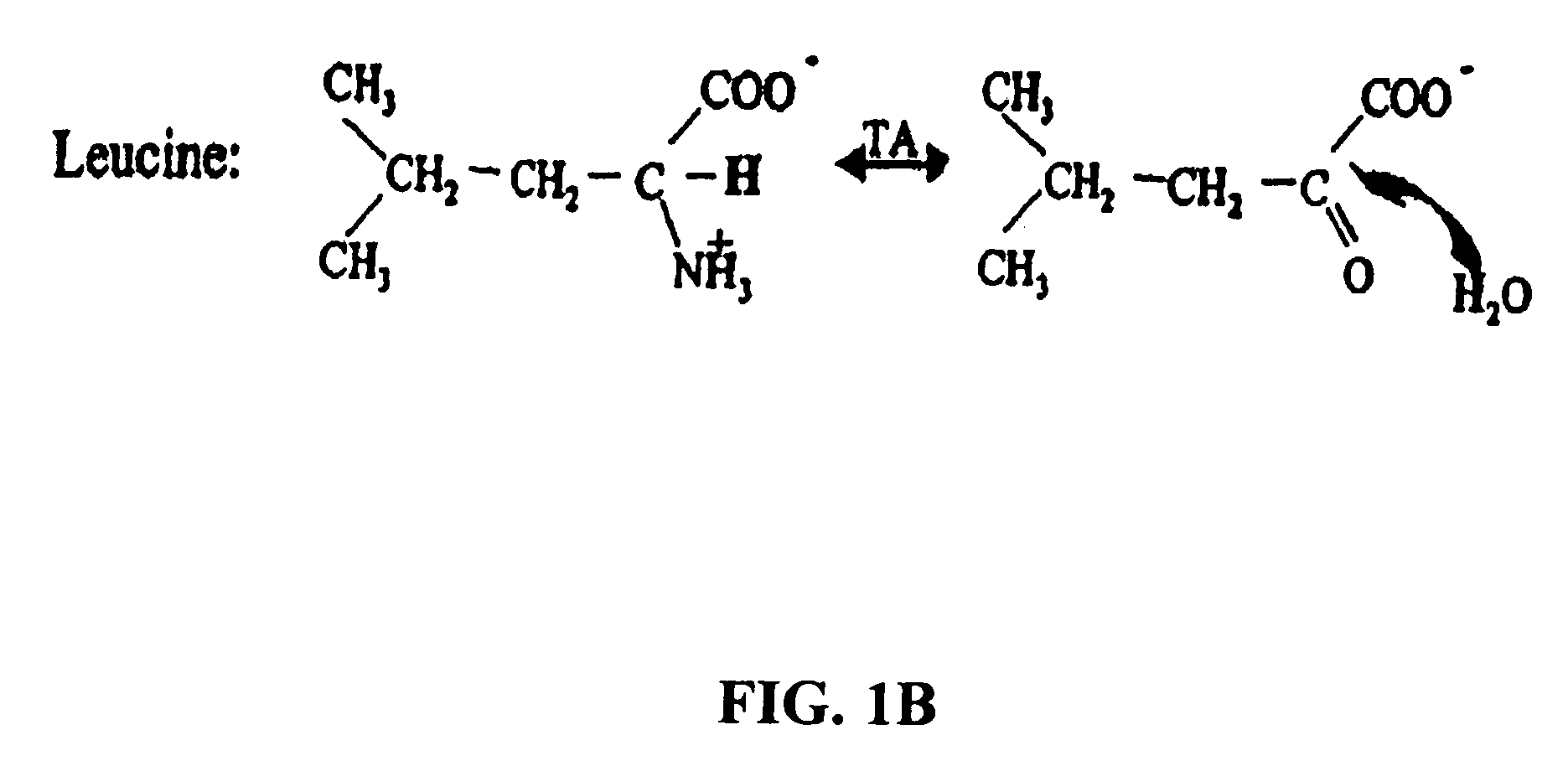
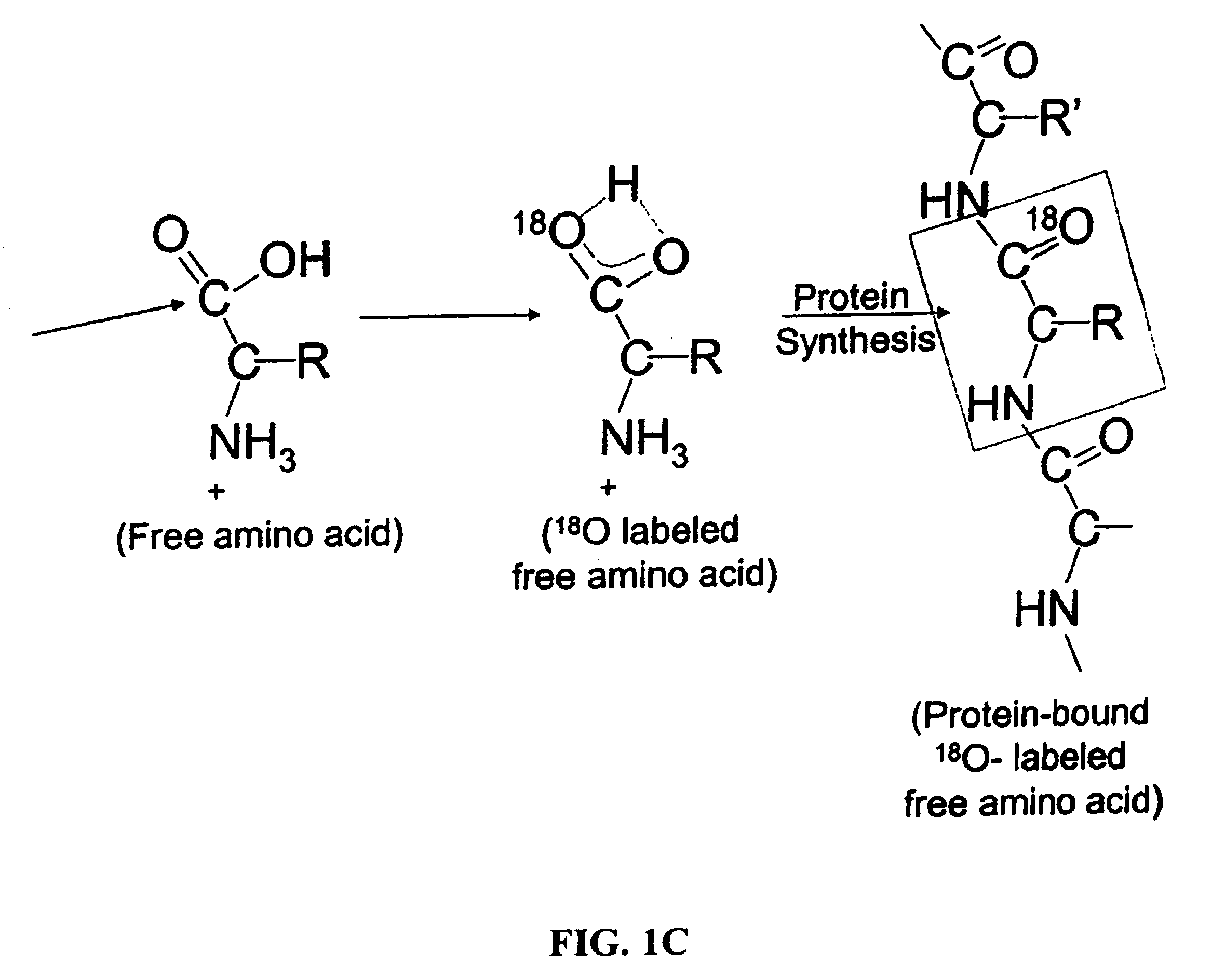
Patents
Literature
32 results about "Drugs trials" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
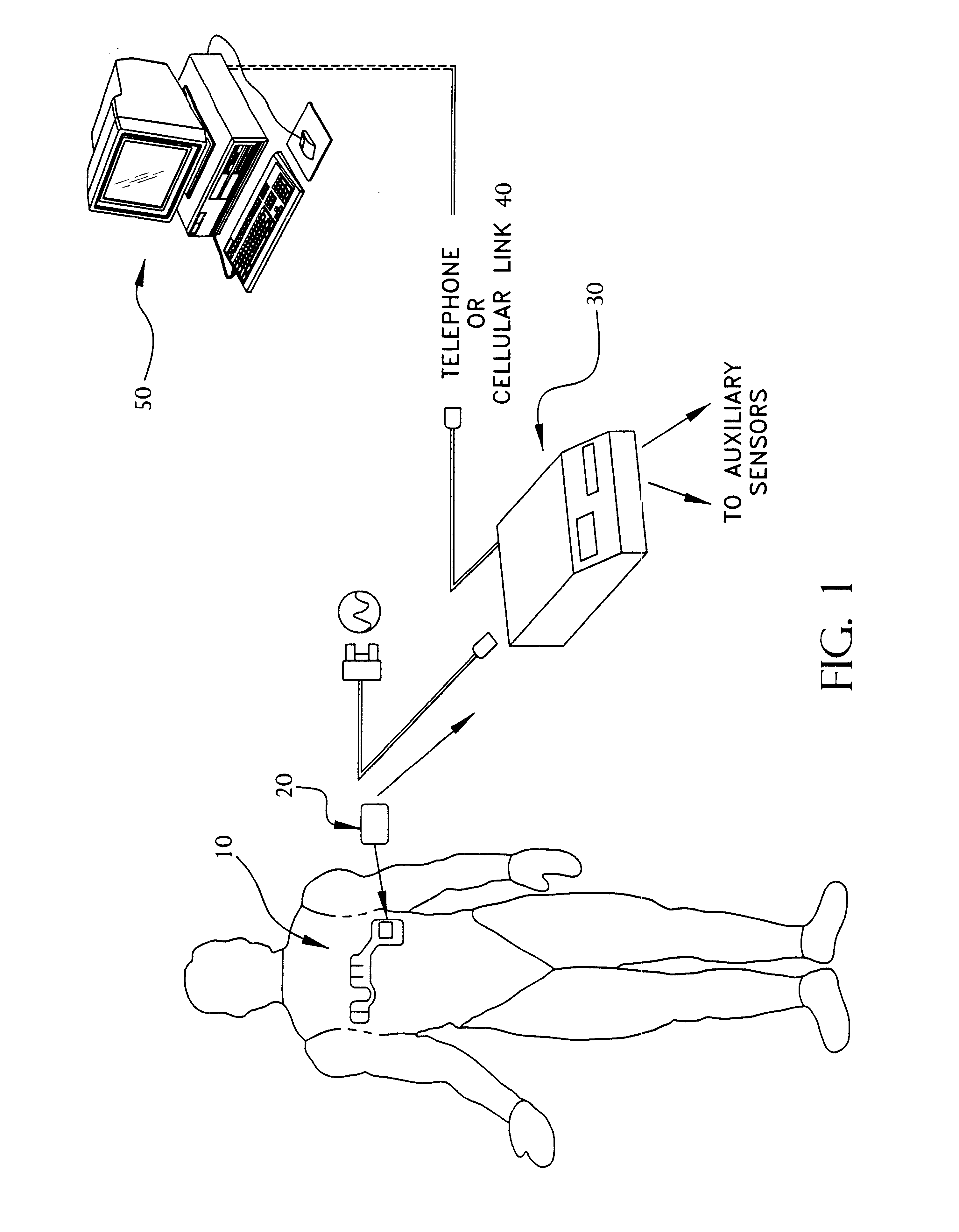
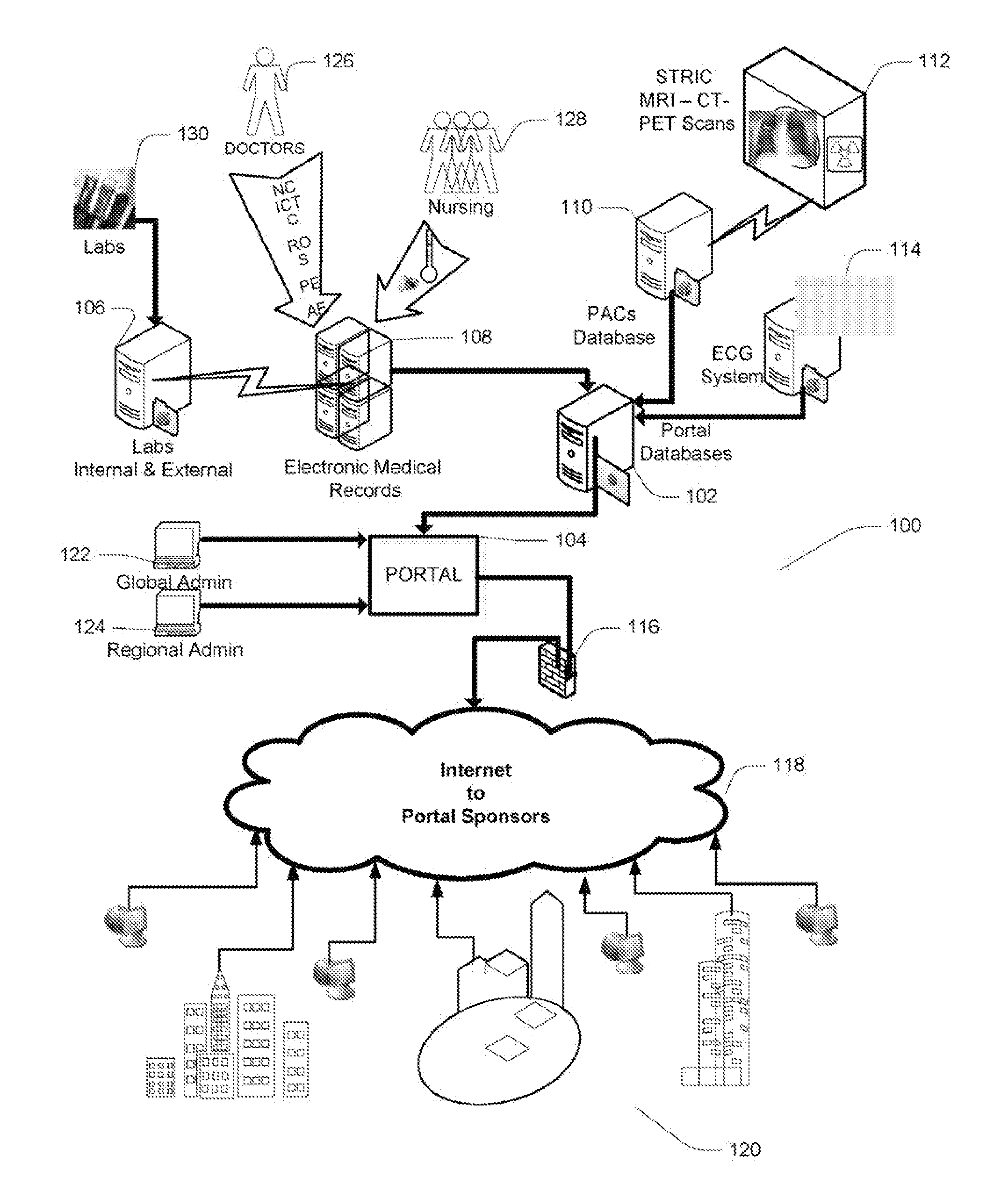
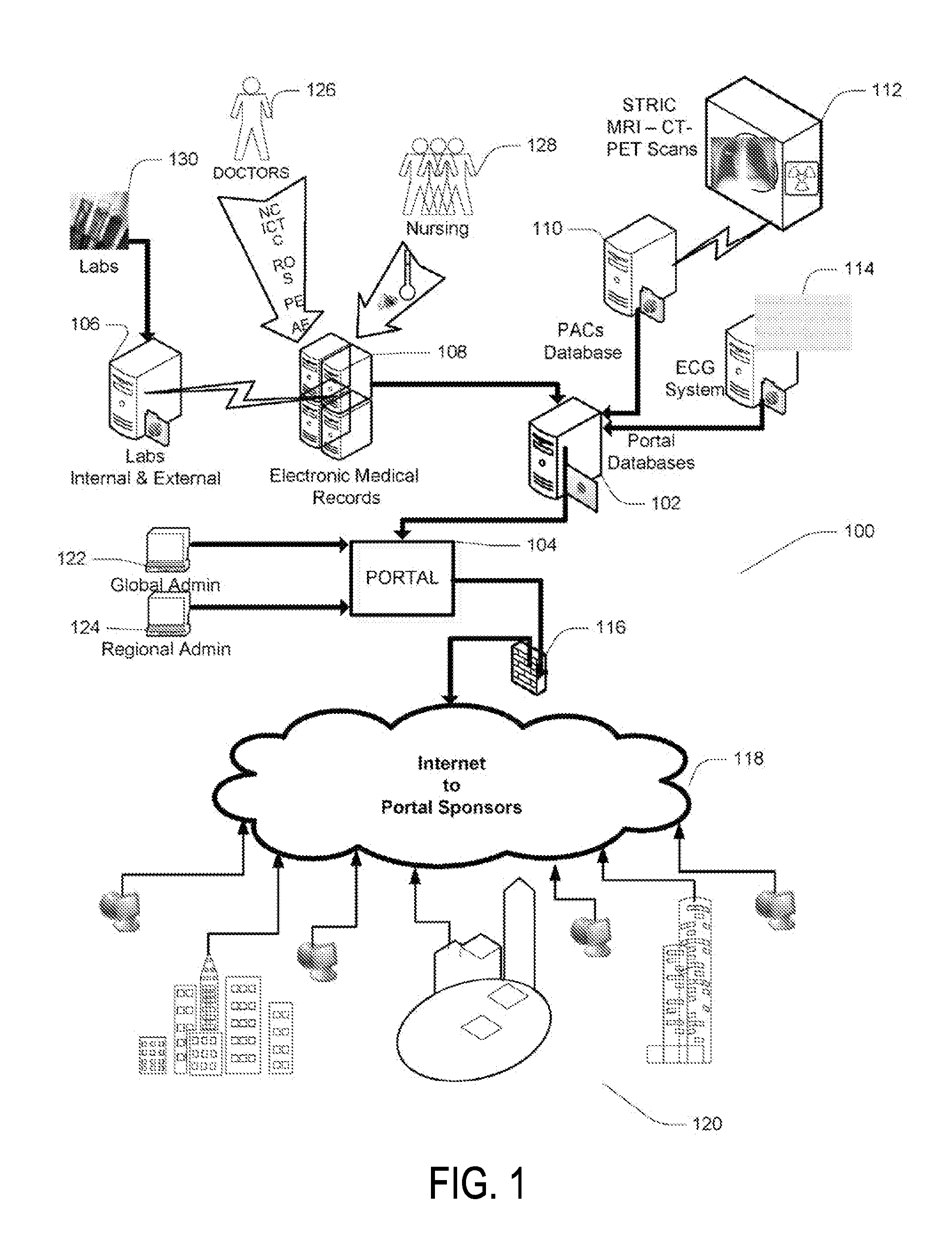
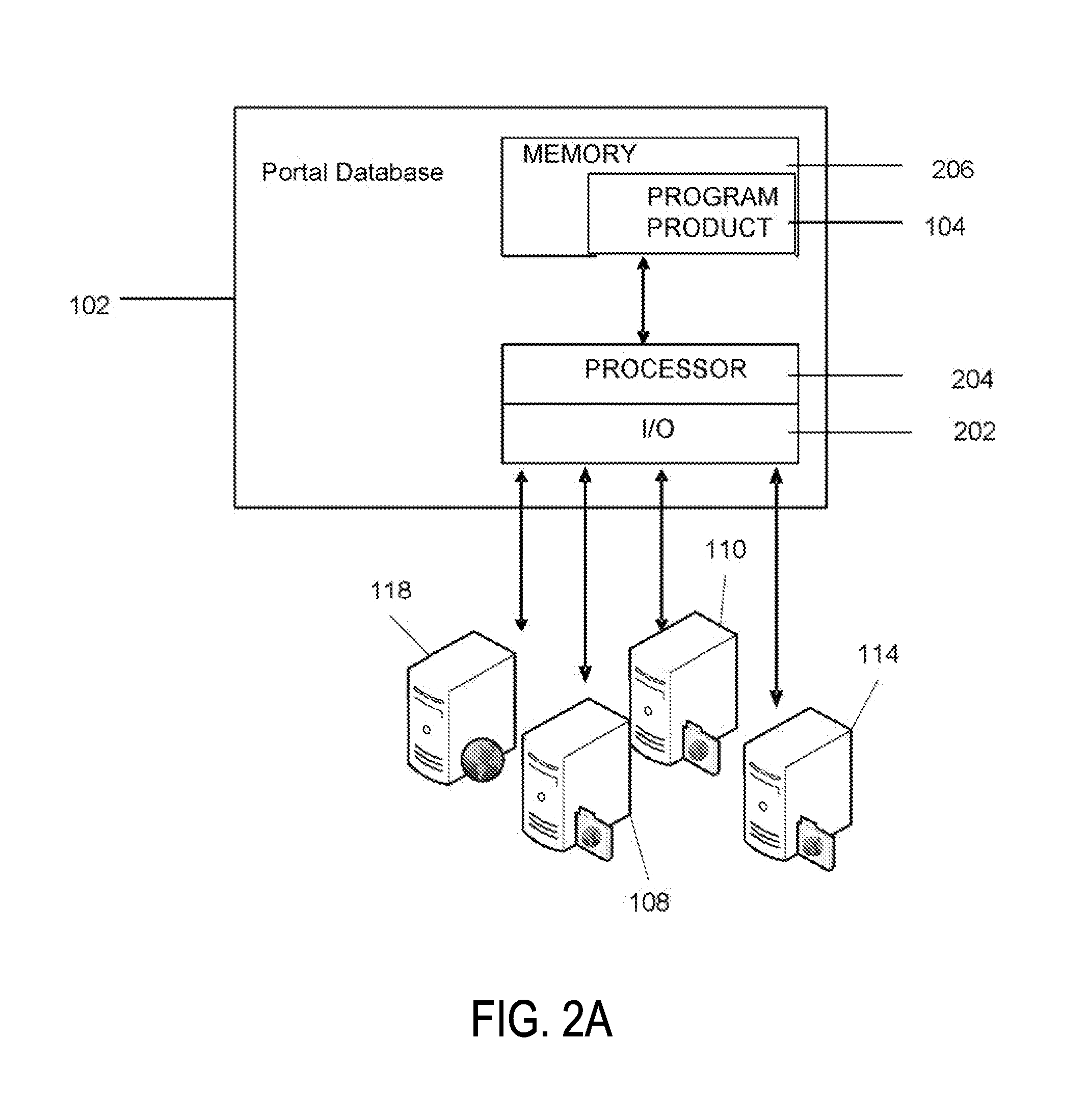
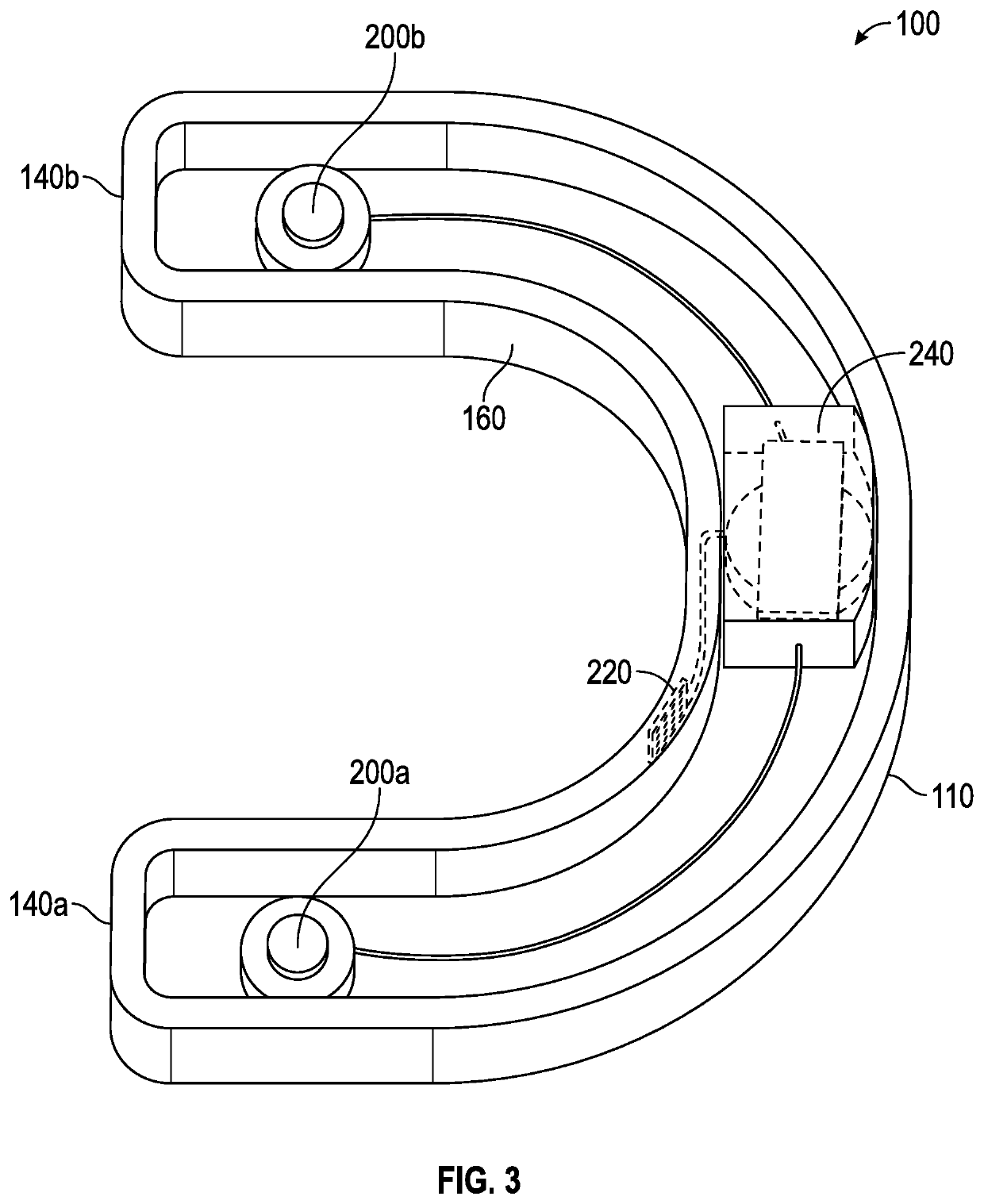
Portable remote patient telemonitoring system
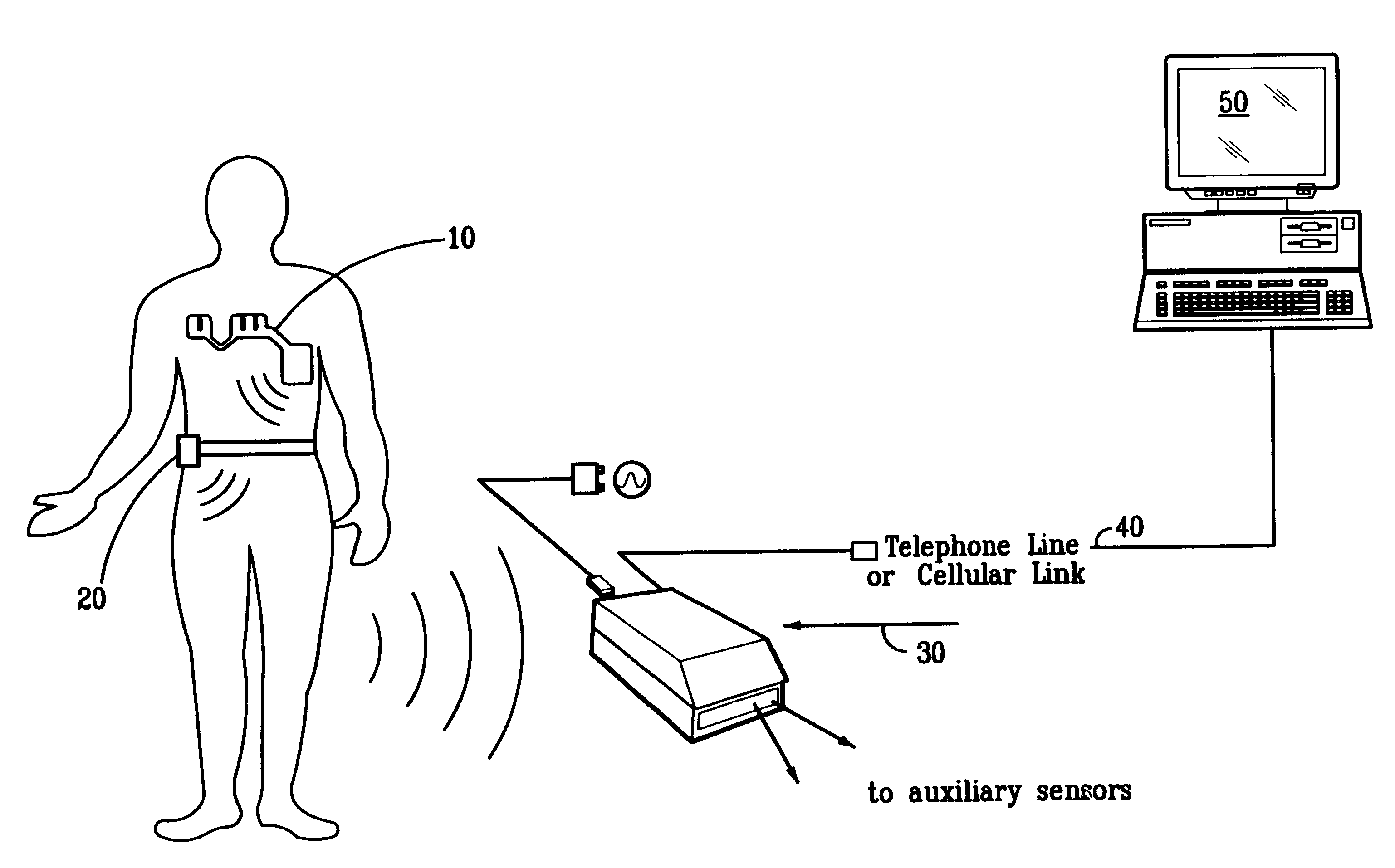
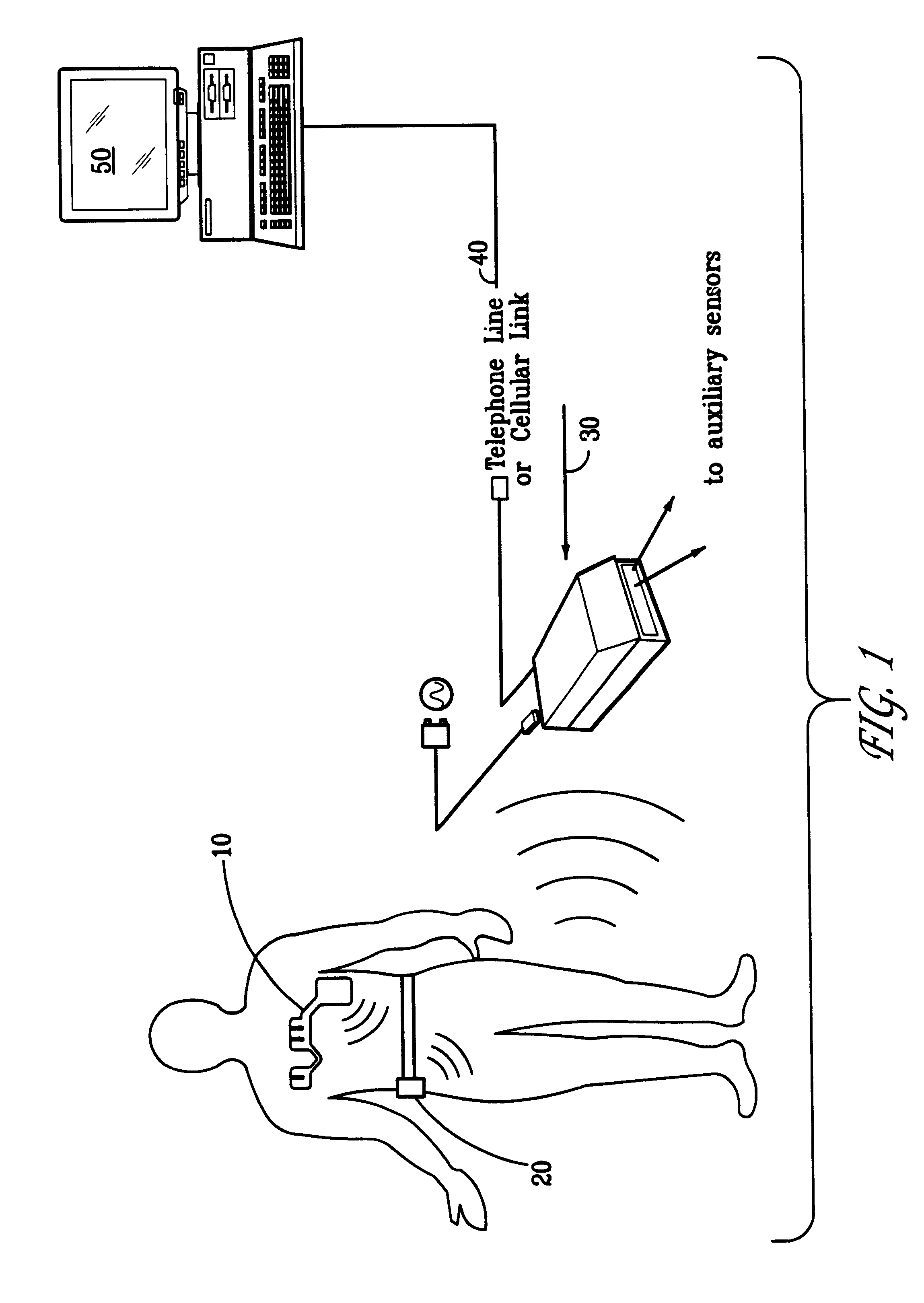
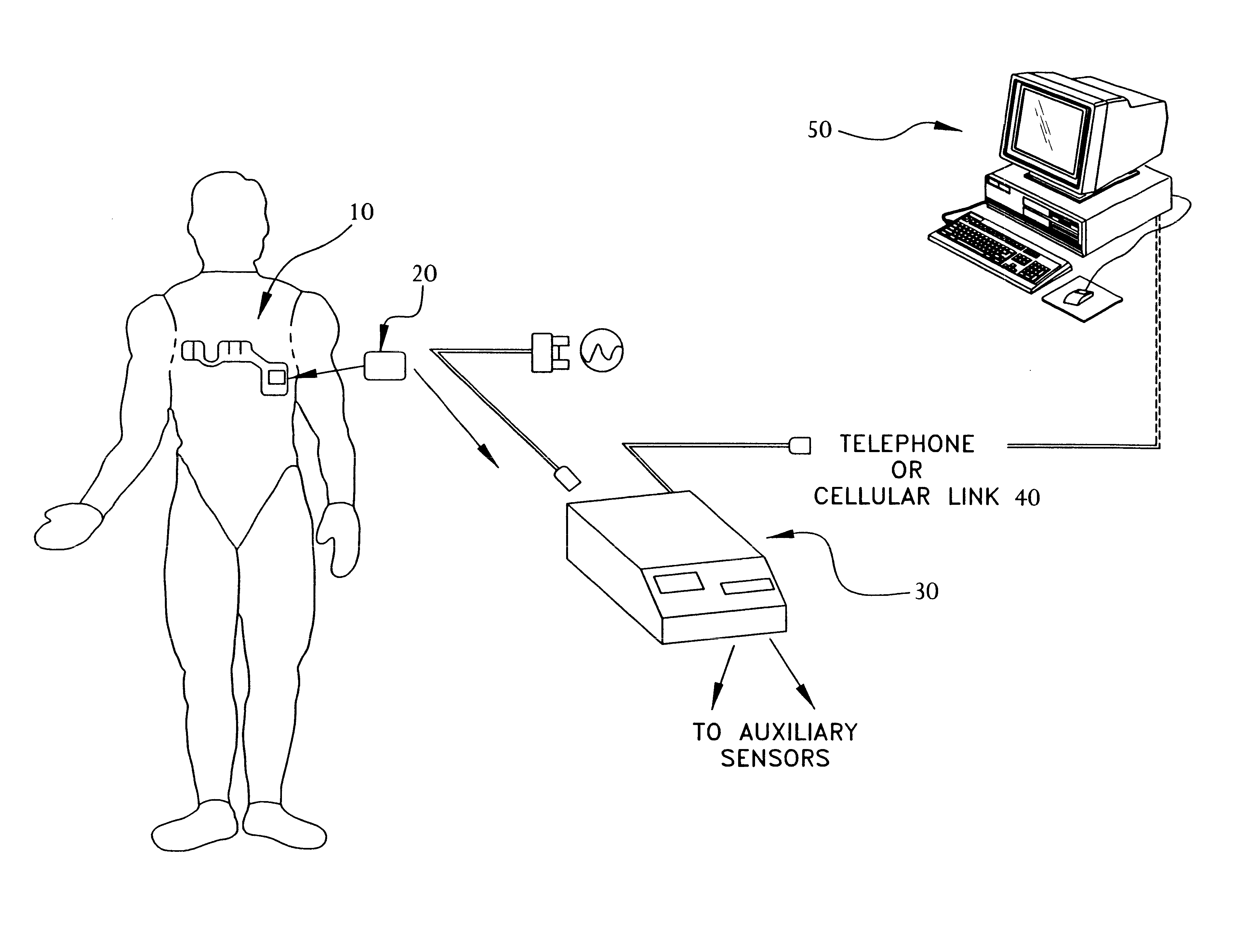
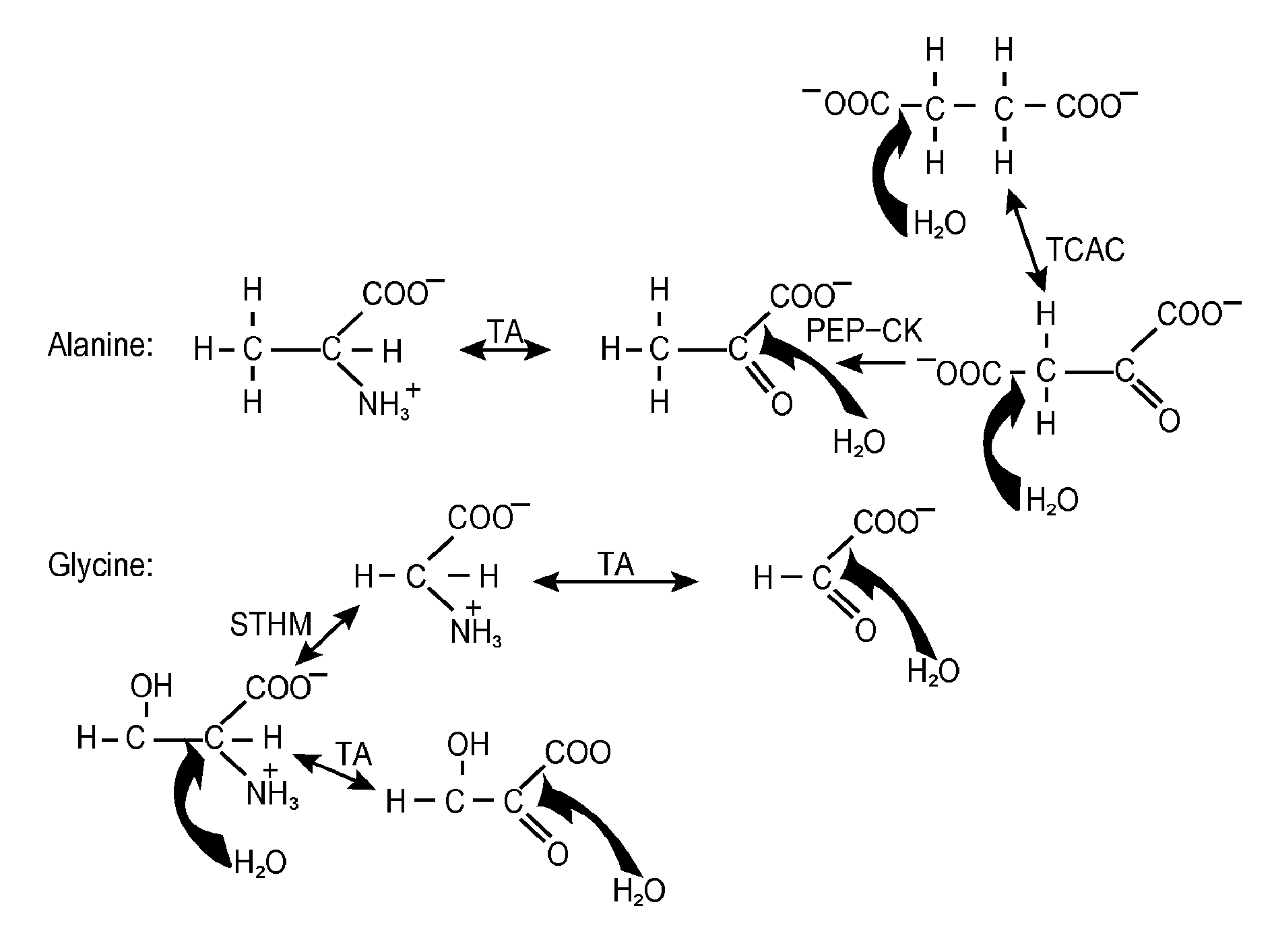
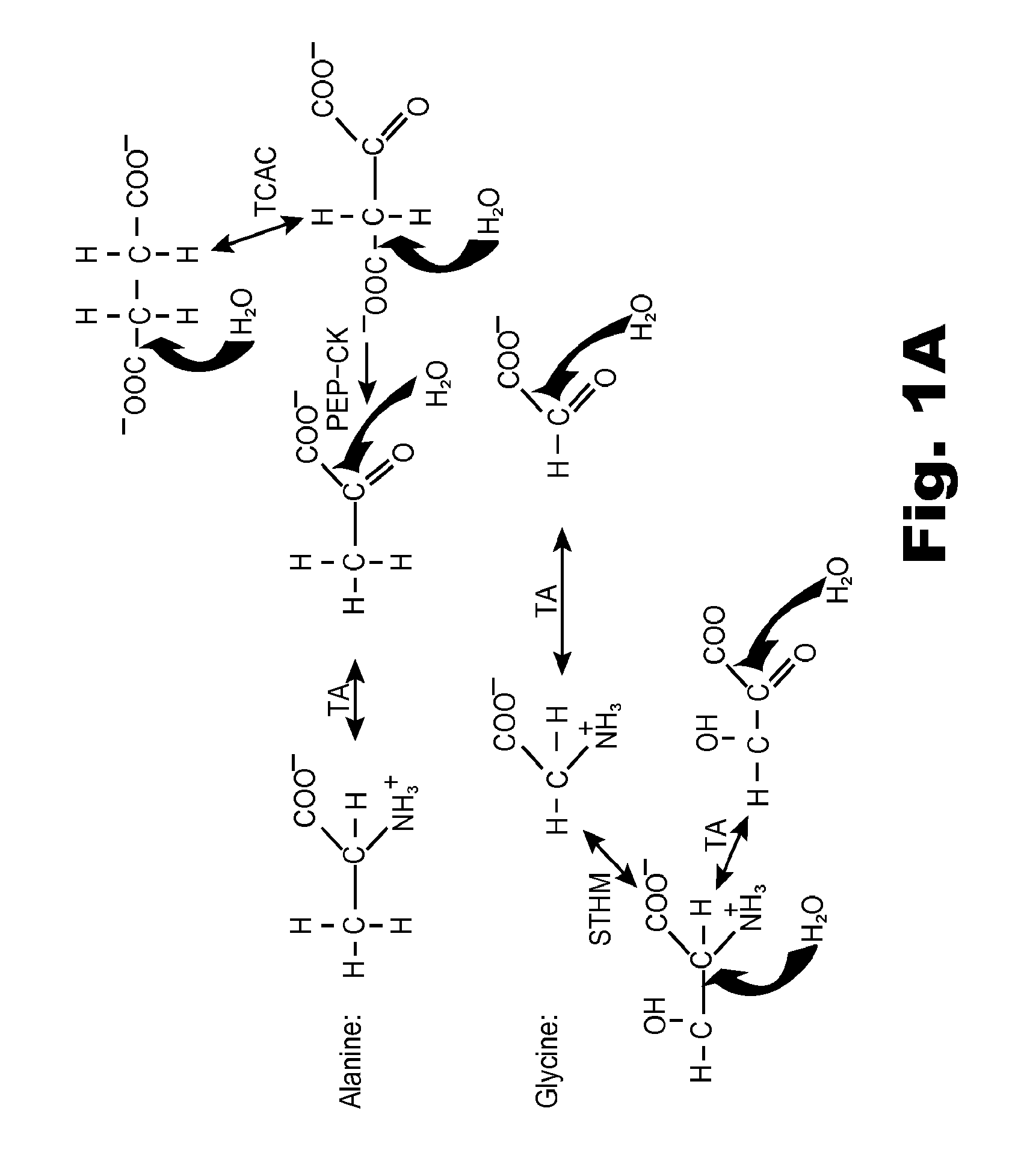
A system and method for monitoring vital signs and capturing data from a patient remotely using radiotelemetry techniques. The system is characterized by a cordless, disposable sensor band with sensors form measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and transmission circuitry for the detection and transmission of vital signs data of the patient. A small signal transfer unit that can either be worn by the patient, e.g., on his or her belt, or positioned nearby receives data from the sensor band, which it then forwards by e.g., radio transmission to a base station that can be located up to 60 meters away. The base station receives data transmissions from the signal transfer unit and is designed to connect to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Patient safety is enhanced by the ability of the base station to compare clinical data, e.g., ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. Such events are indicated to the physician and could be indicated to the patient by reverse transmission to the signal transfer unit. A remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. The system of the invention has useful application to the collection of patient clinical data during drug trials and medical testing for regulatory approvals as well as management of patients with chronic diseases.
Owner:CLEARPATH PARTNERS
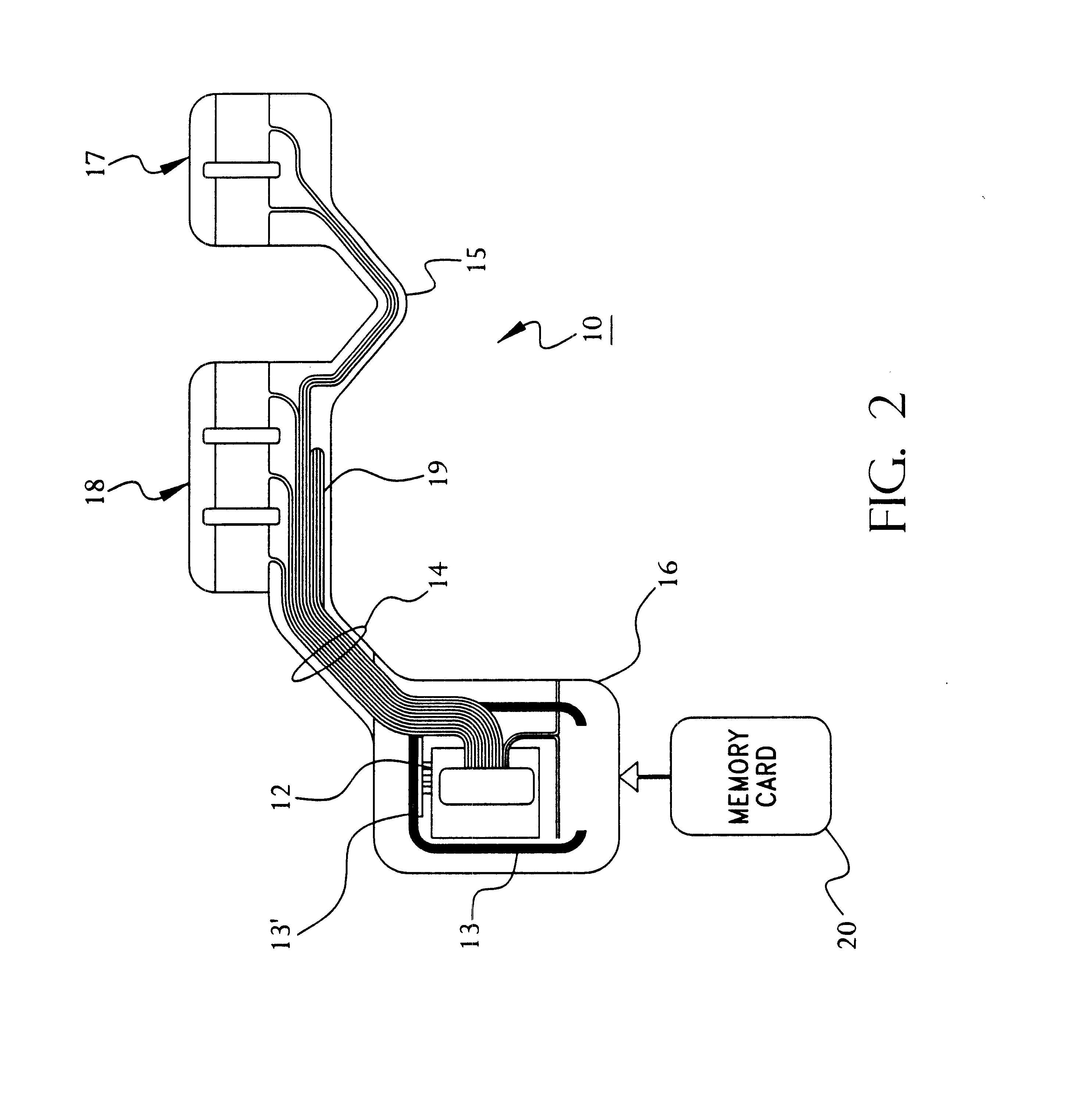
Portable remote patient telemonitoring system using a memory card or smart card
InactiveUS6454708B1Low costIncrease the number ofSurgeryRespiratory organ evaluationSmart cardFull waveform
A system and method for monitoring health parameters and capturing data from a subject. The system is characterized by a cordless, disposable sensor band with sensors for measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and a connector which accepts a memory card or a smart card for storage of the measured data. After a predetermined period of time, such as when the sensor band is removed, the memory card or smart card is removed and inserted into a monitoring device which reads the stored health parameter data of the subject. The monitoring device includes a base station that includes a memory / smart card reader and is connected to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Subject safety is enhanced by the ability of the base station to compare clinical data, e.g. ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. The remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. In alternative embodiments, a smart card includes the sensor band's electronics and / or signal transmission circuitry in conjunction with a portable data logger so that the electronics may be reused from one disposable sensor band to the next without limiting the patient's range of movement. The system of the invention has useful application to the collection of subject clinical data during drug trials and medical testing for regulatory approvals as well as management of subjects with chronic diseases.
Owner:CLEARPATH PARTNERS
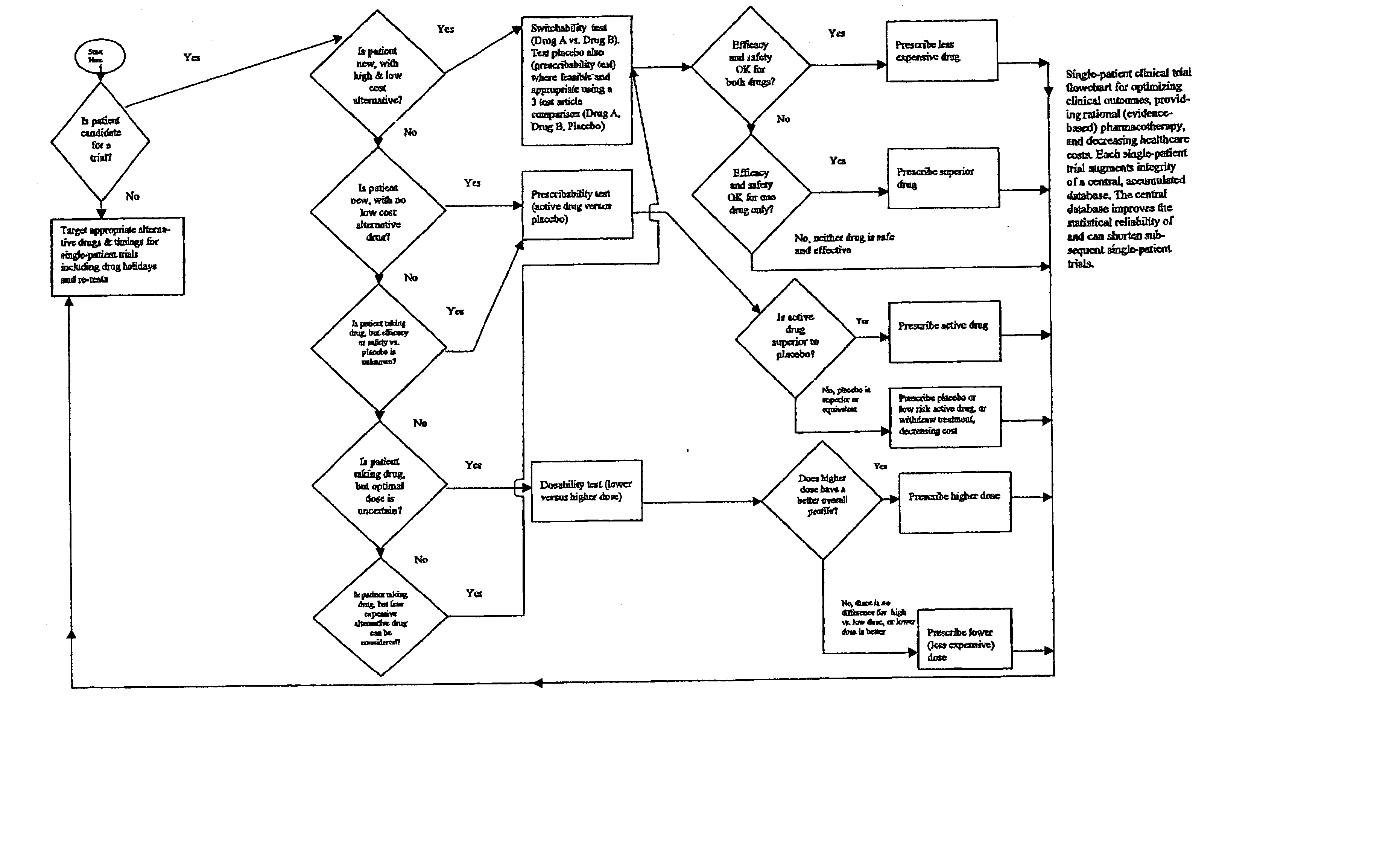
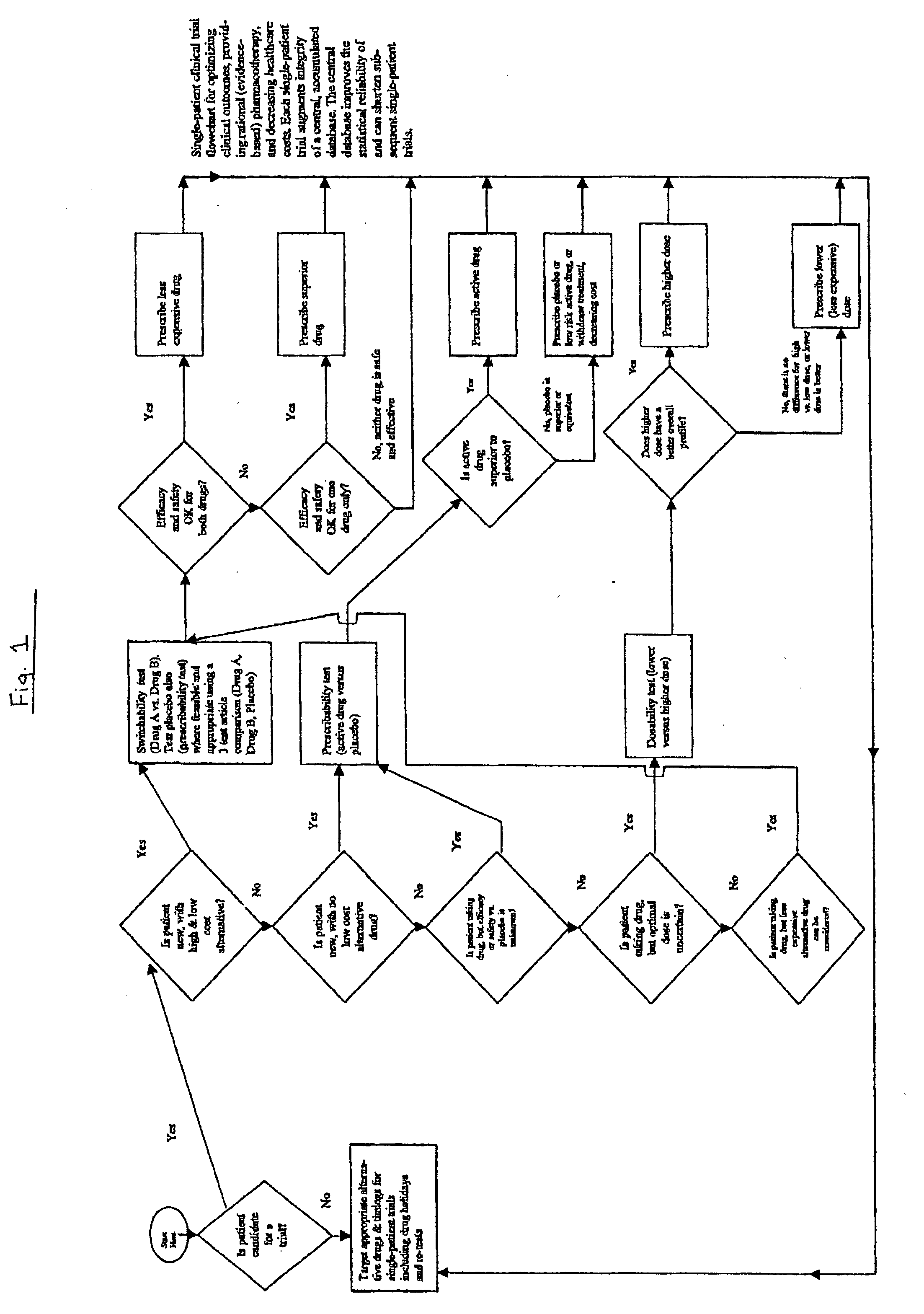
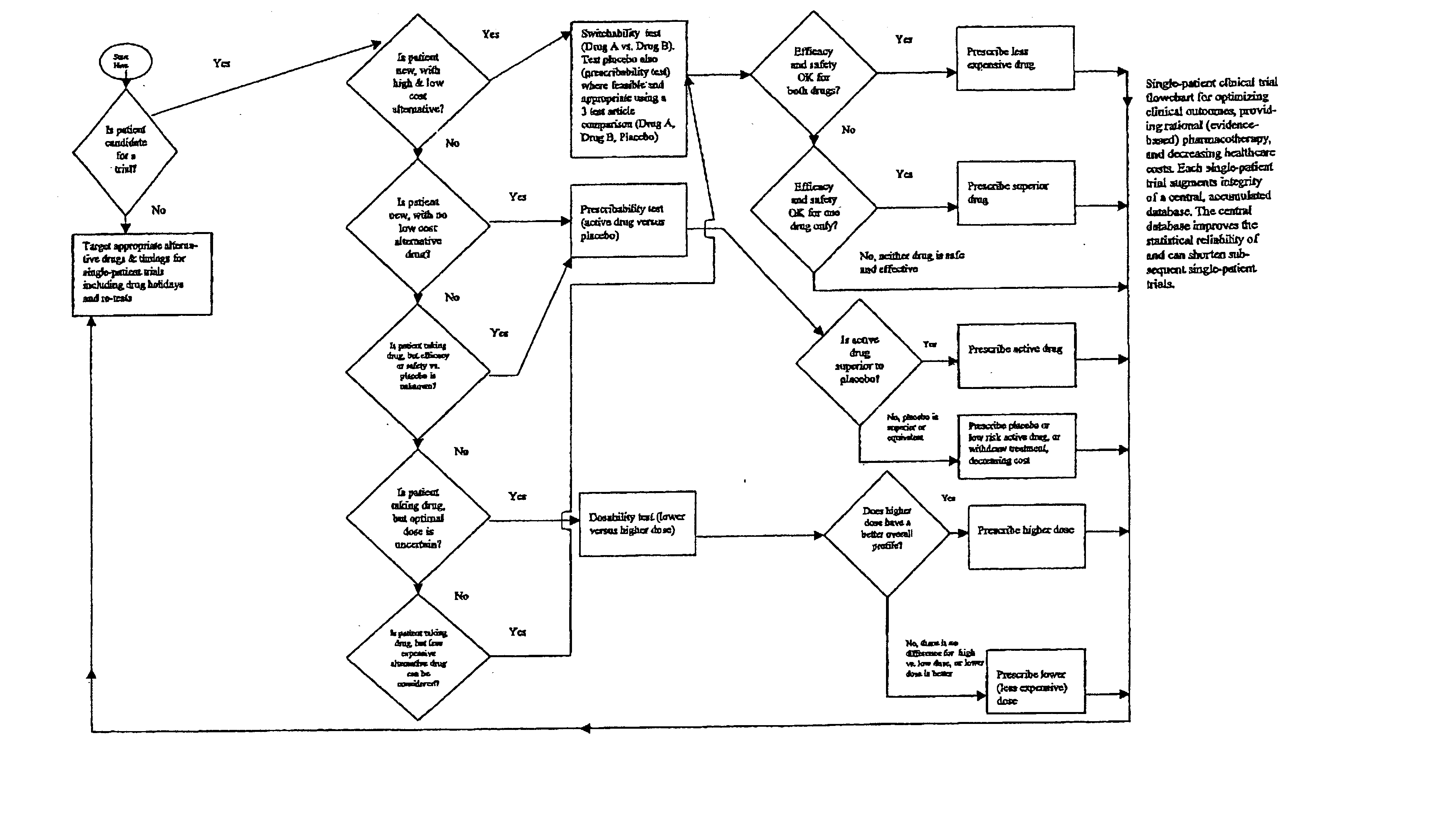
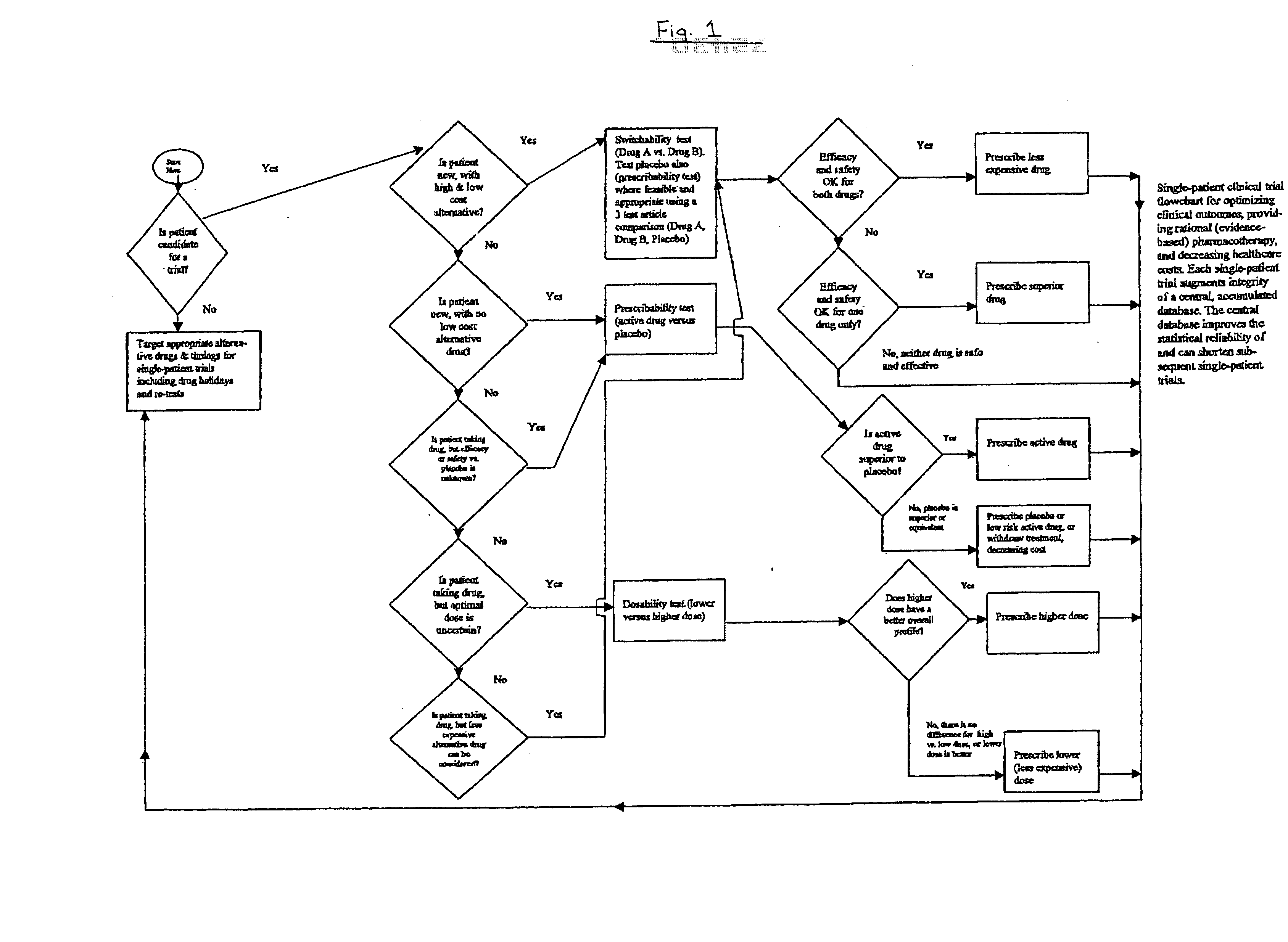
Single-patient drug trials used with accumulated database: risk of habituation
InactiveUS20020032581A1AppropriatenessImprove statistics performancePhysical therapies and activitiesDrug and medicationsHabituationDrug trial
A method of evaluating and / or optimizing clinical outcomes and providing rational pharmacotherapy in an individual or animal requiring chronic drug therapy is provided.
Owner:OPT E SCRIP
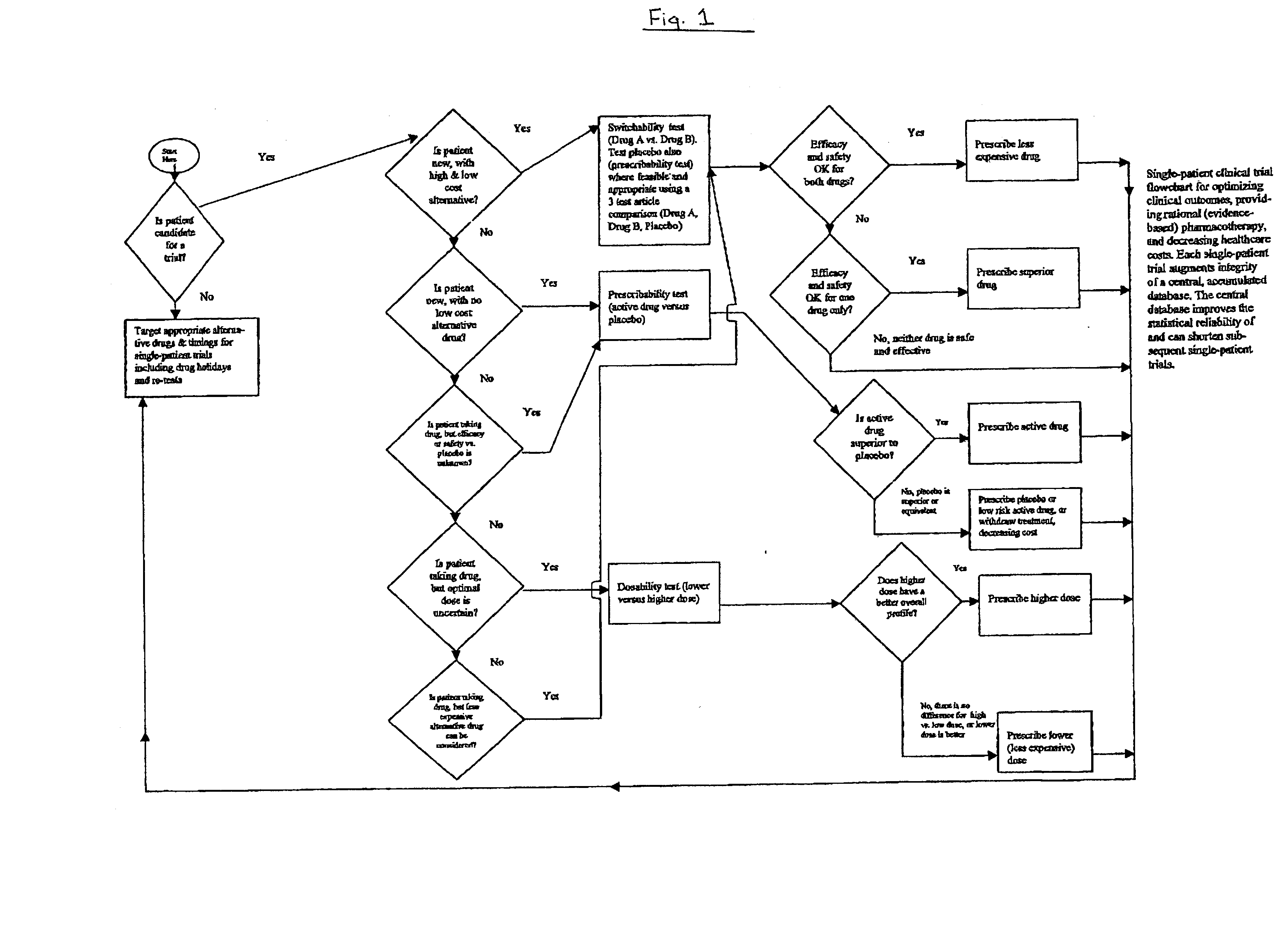
Single-patient drug trials used with accumulated database: flowchart
InactiveUS20020192159A1AppropriatenessImprove statistics performanceCompounds screening/testingData processing applicationsMedicineDrug trial
A method of evaluating and / or optimizing clinical outcomes and providing rational pharmacotherapy in an individual or animal requiring chronic drug therapy is provided.
Owner:OPT E SCRIP
Single-patient drug trials used with accumulated database: genomic markers
InactiveUS20020038310A1AppropriatenessImprove statistics performancePhysical therapies and activitiesDrug and medicationsMedicineDrug trial
Owner:OPT E SCRIP
Managing research data for clinical drug trials
InactiveUS20140142961A1High transparencyExtension of timeData processing applicationsLaboratory analysis dataClinical researchLibrary science
A machine, computer program product, and computer-implemented method for performing a process of managing clinical research data by collating a plurality of clinical patient records into a database system and a process of allowing particular clinical users to access the clinical research records.
Owner:SOUTH TEXAS ACCELERATED RES THERAPEUTICS
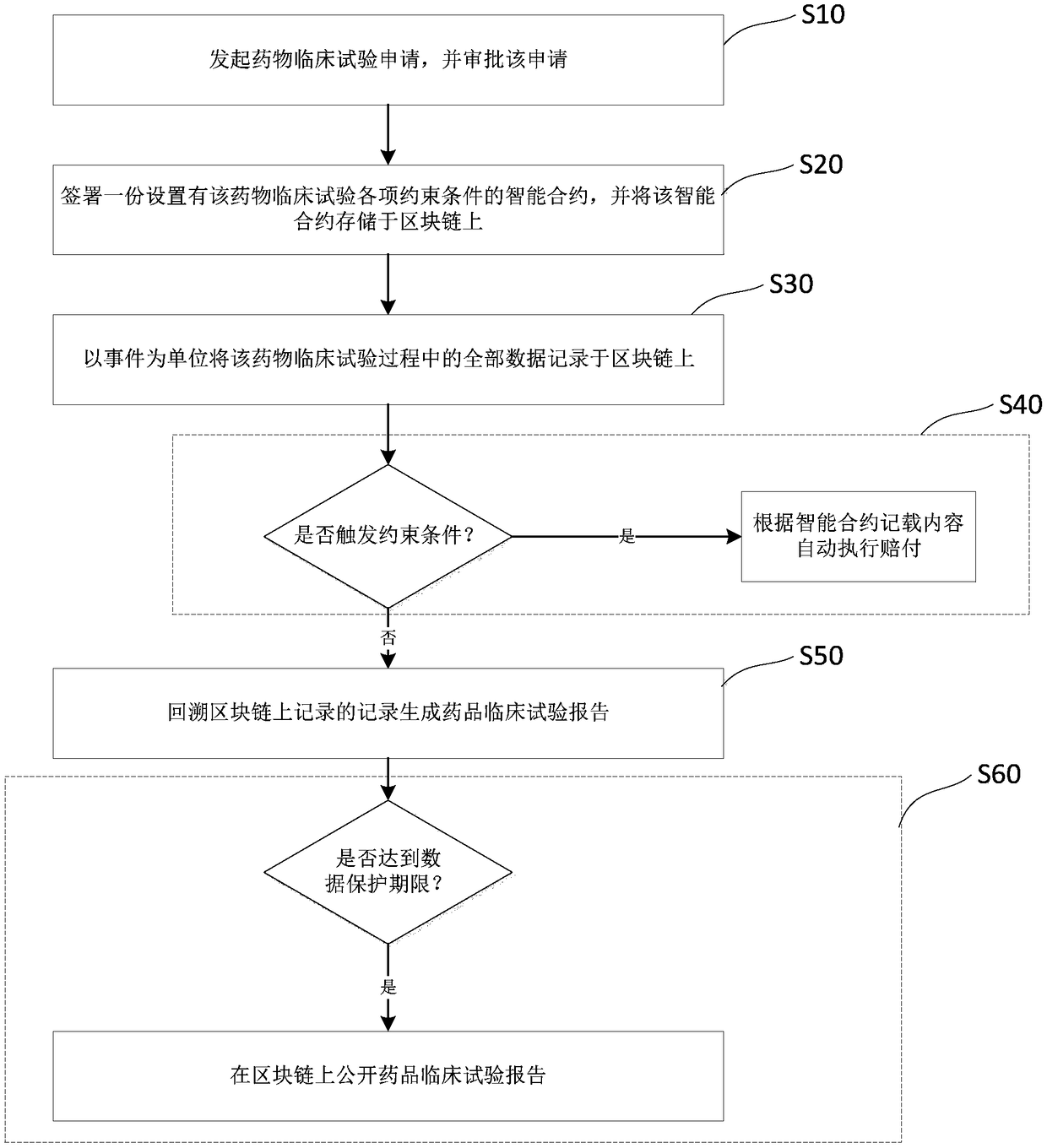
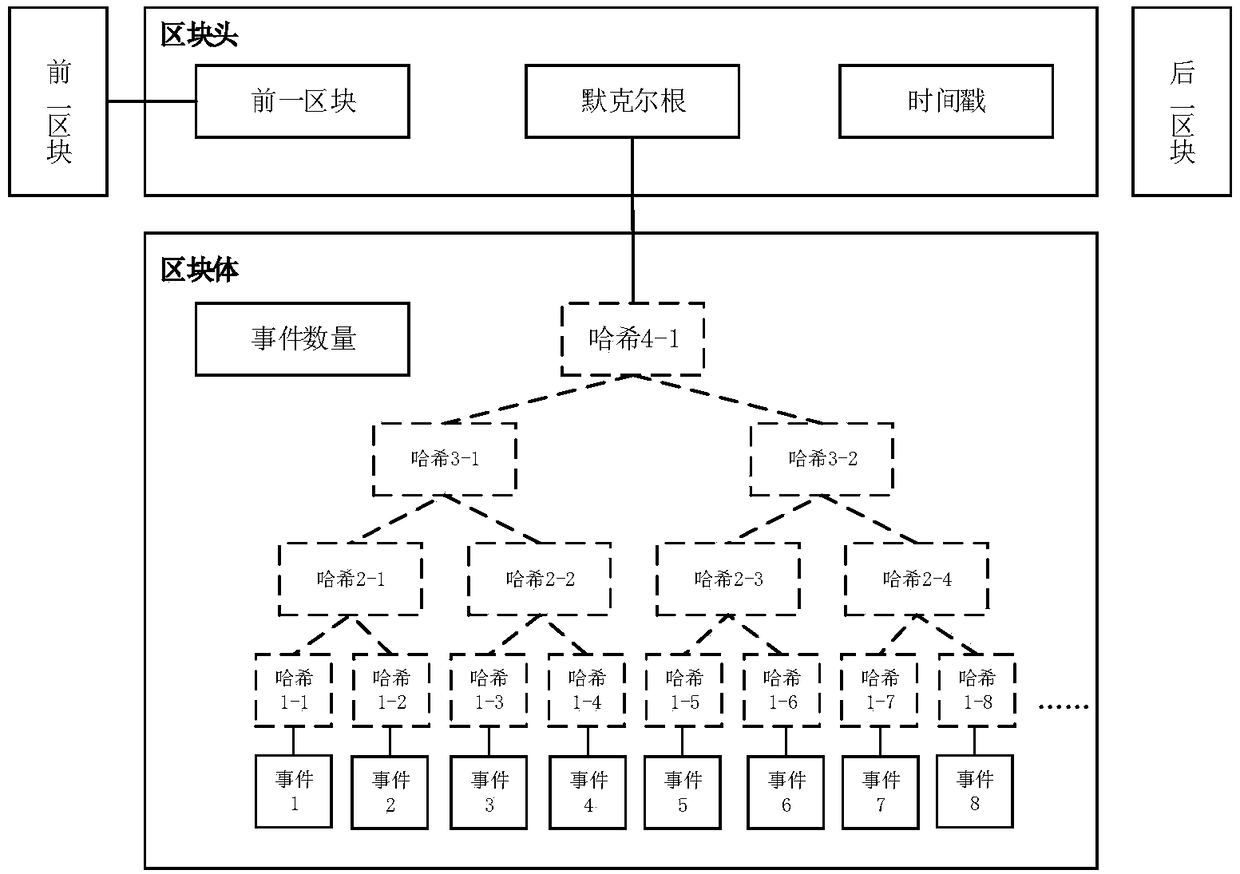
Drug clinical trial monitoring method and system based on block chain, device and medium
InactiveCN109065101AGuaranteed to be true and reliableProtection of rights and interestsDrug referencesSpecial data processing applicationsData authenticityEvent trigger
The invention discloses a drug clinical trial monitoring method and system based on the block chain, a device and a medium and relates to the technical field of the drug clinical trial. The block chain technology is introduced, test records are maintained jointly by participants in the drug trial and stored in a block chain in a chronological order through the chain structure, through use of the block chain, characteristics can be only added and are non-tamperable and deleteable, and data authenticity and validity are guaranteed; rights of subjects are better protected through the intelligentcontract technology, an informed consent is stored in the block chain in the form of an intelligent contract, during the test, the physiological data of the subjects are obtained in real time throughdocking with the medical device, once the event triggering a contract suspension clause is monitored, payment is automatically executed according to the contract terms; through the decentralized storage idea, defects including large cost of data storage space, incomplete privacy protection and weak anti-attack ability existing in a centralized drug test management system in the prior art are solved.
Owner:INST OF SOFTWARE APPL TECH GUANGZHOU & CHINESE ACAD OF SCI
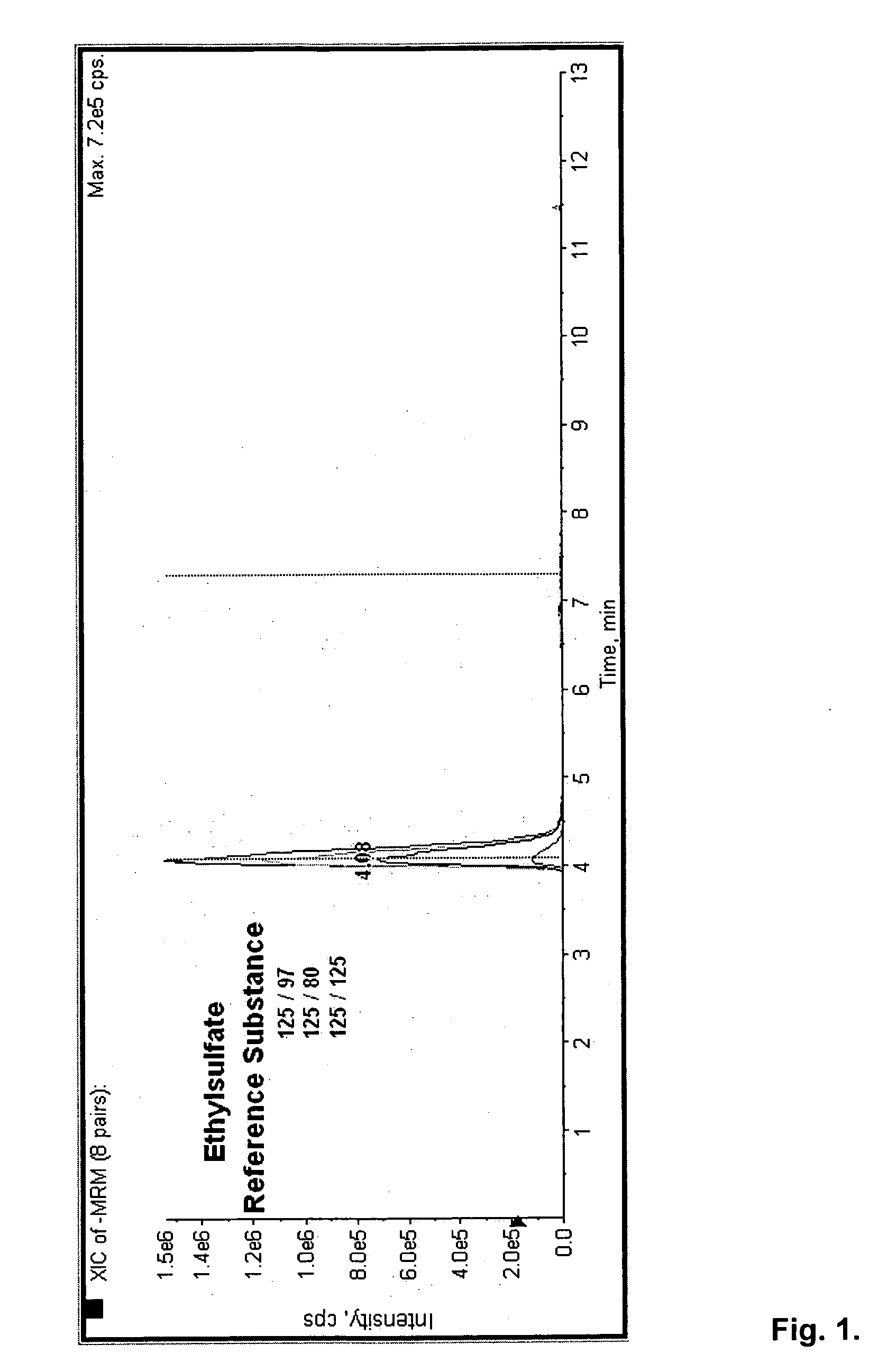
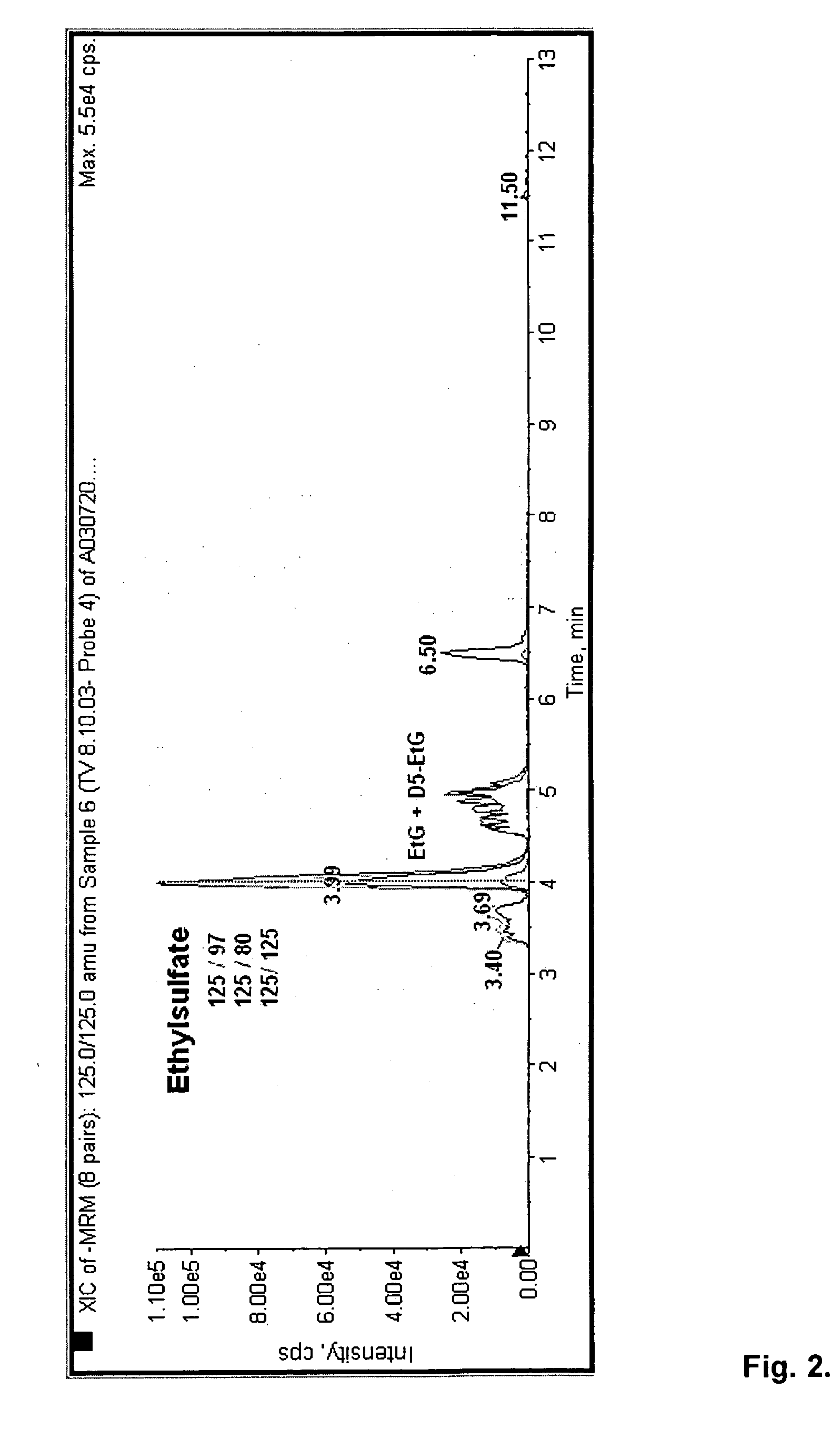
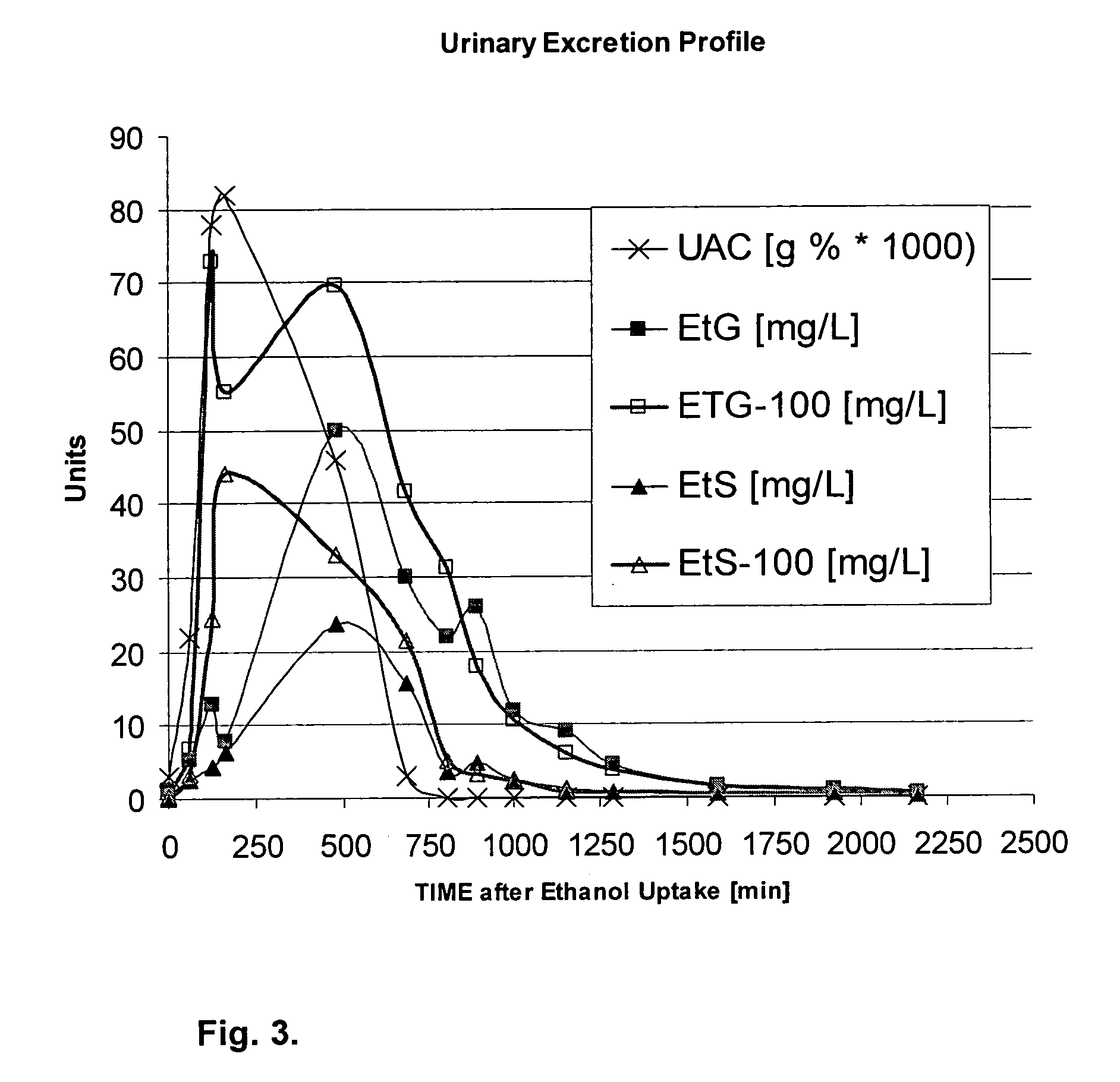
Direct ethanol metabolite ethyl sulfate as an useful diagnostic and therapeutic marker of alcohol consumption
InactiveUS20060084134A1Microbiological testing/measurementDisease diagnosisTime spectrumAlcohol intoxication
Accurate self report strategies, biological state markers, combinations of alternative biomarkers, and combinations of biomarkers and self reports capable of monitoring alcohol consumption with a high sensitivity and specificity over a broad time spectrum are disclosed. In particular, the use of ethyl sulfate (monoethylsulfate, molecular weight 126 g / mol, C2H5SO4H) to elucidate ethanol intake is described in the context of screening monitoring in various settings, e.g. a) after liver transplantation b) methadone maintenance patients with hepatitis C and comorbid excessive alcohol use c) underage drinking d) rehabilitation programs for alcoholics motivational feedback to improve knowledge on drinking patterns differentiating moderate / social drinking from problematic / harmful drinking differential diagnosis (e.g. elevated transaminases) evaluating treatment programs and drug trials elucidating the role of neuropsychological impairment following alcoholisation (i.e. hangover state) which plays a major role in accidents, disclosing recent drinking in social drinkers in risky situations (driving, workplaces, pregnancy (fetal alcohol syndrome (FAS)), and general monitoring.
Owner:WURST FRIEDRICH MARTIN
Clinical trial phase simulation method and clinical trial phase simulator for drug trials
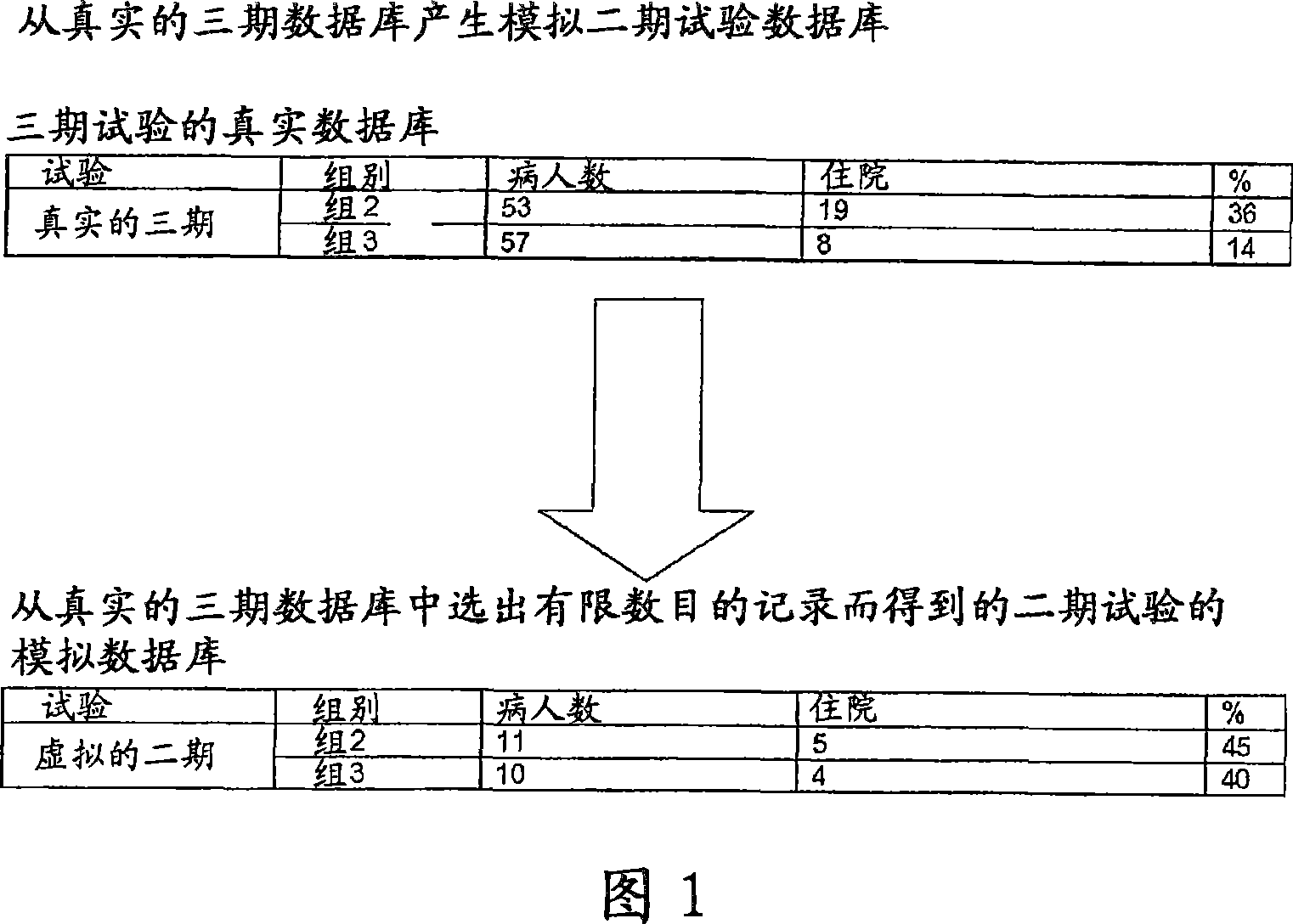
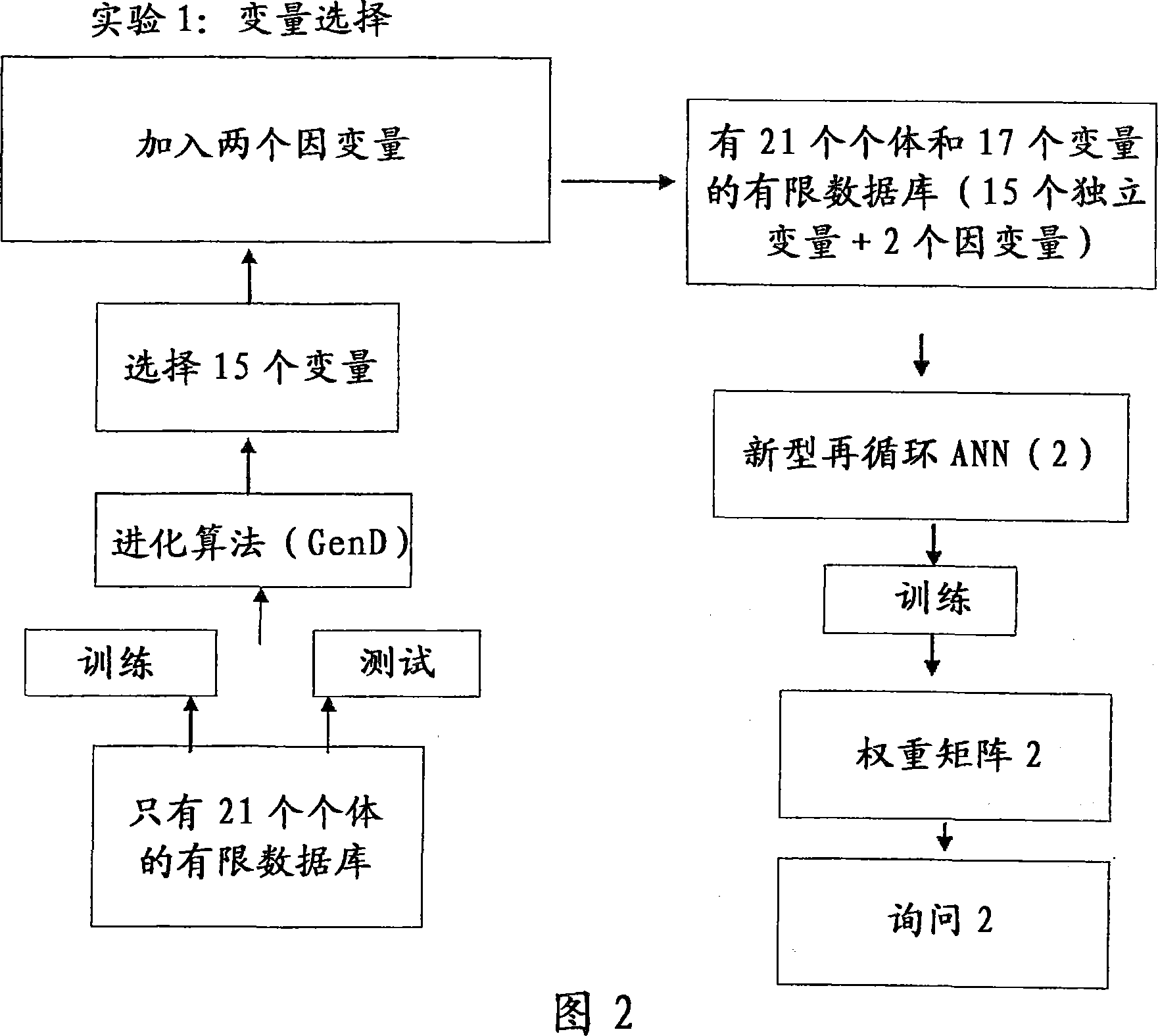
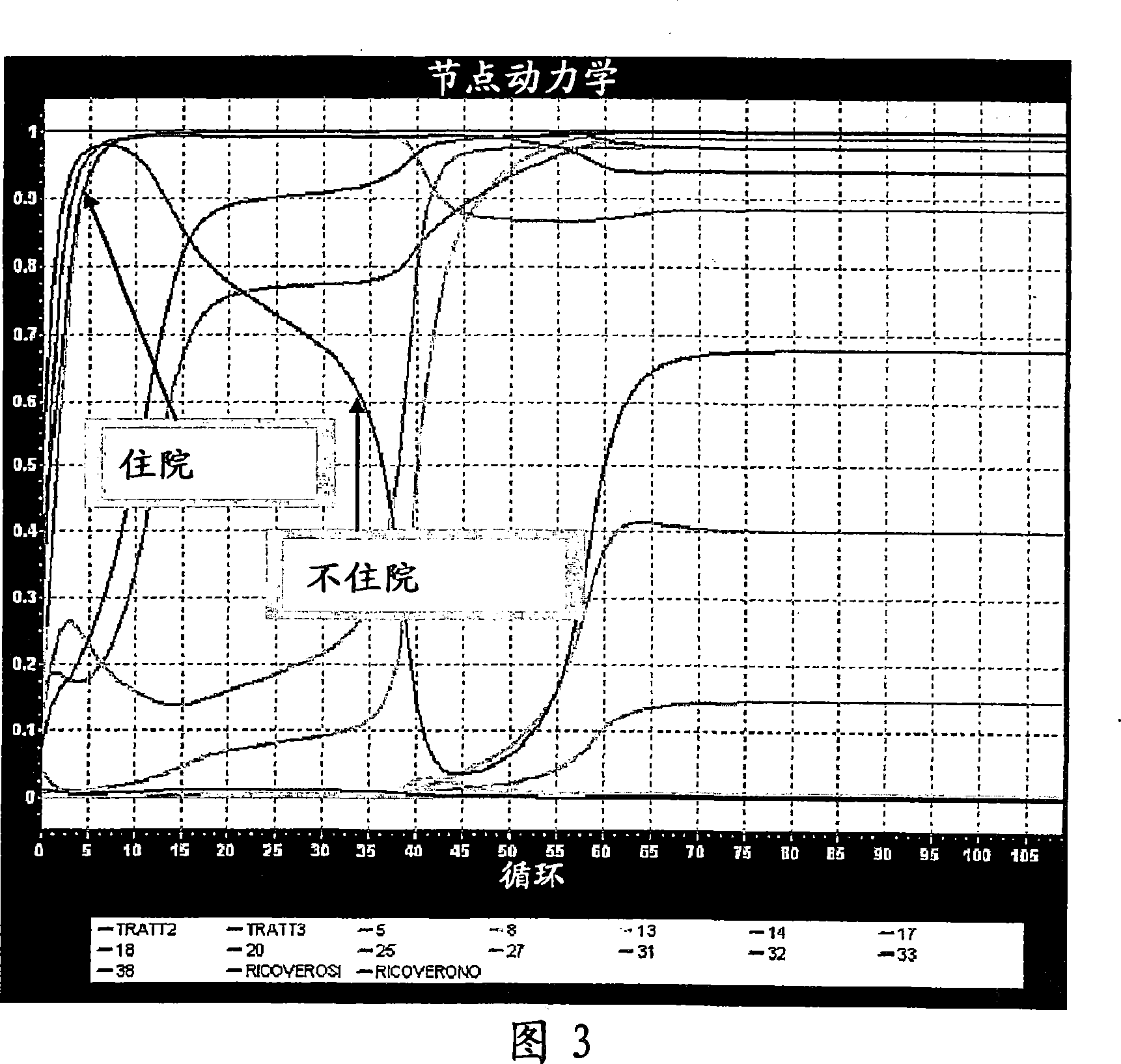
InactiveCN1977270AMedical simulationComputer-assisted medicine prescription/deliveryNerve networkMedicine
A clinical trial phase simulation method for drug trials, which method allows to predict the trend of the results of a clinical trial phase of a drug with the steps of providing a database comprising for each of a certain number of individuals a predefined number of independent variables each of which corresponds to a certain clinical parameter relevant or characteristic for a disease condition against which the drug to be tested is oriented and at least a further independent variable describing the specific treatment to which the individual has been subjected between at least two different treatments one with the said drug and the second with a placebo or with another known drug, the database comprising also for each individuals one or more dependent variables describing the effects of the said treatments; carrying out an input variable selection; adding to the independent variables selected as input variables the dependent variables describing the effects of the treatments; training and validating an artificial neural network with the selected variables as input variables and with the dependent variables; interrogating the said neural network by inputting the values of the variable describing one of the treatments and obtaining as an output the variable values of the effectiveness of the treatment to which the inputted values of the variable of the treatment correspond according to the trained artificial neural network.
Owner:BRACCO IMAGINIG SPA
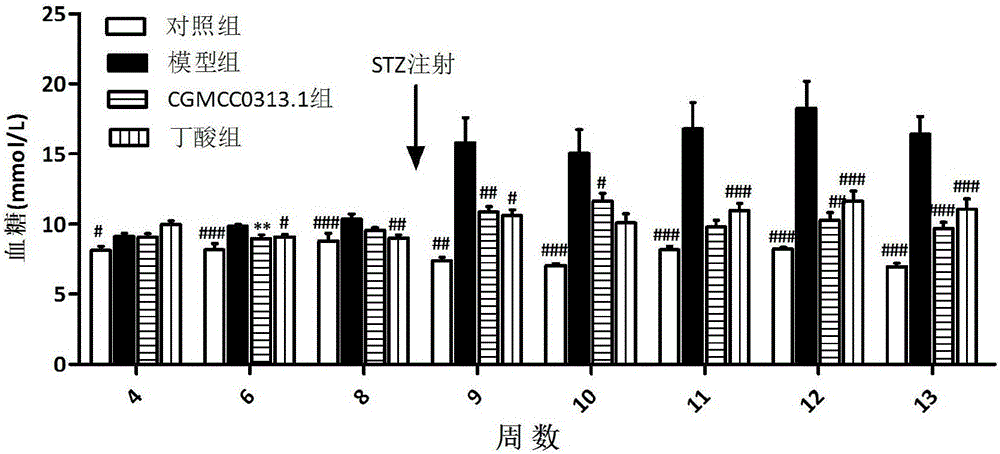
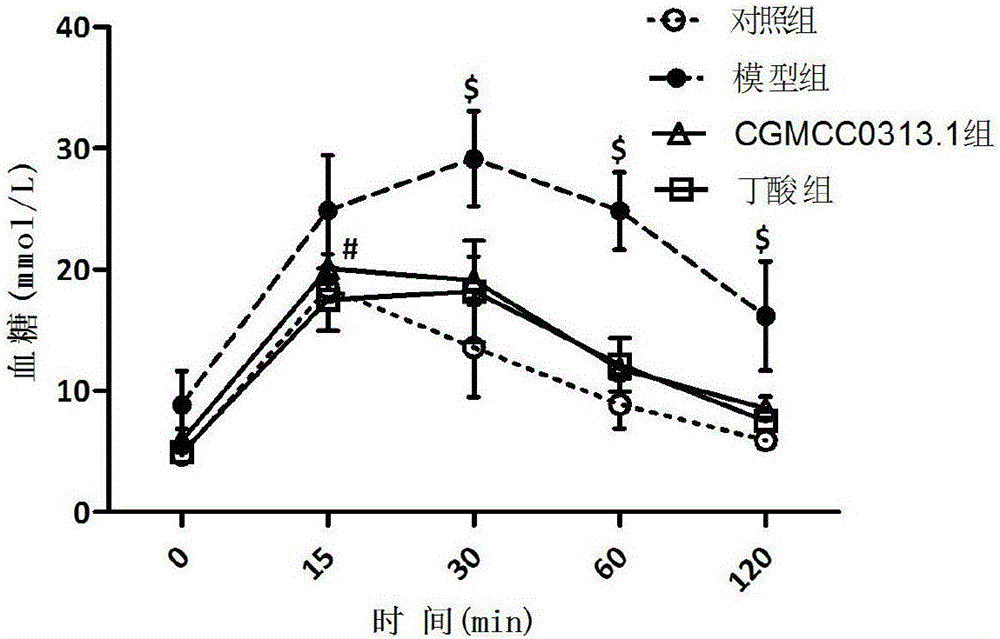
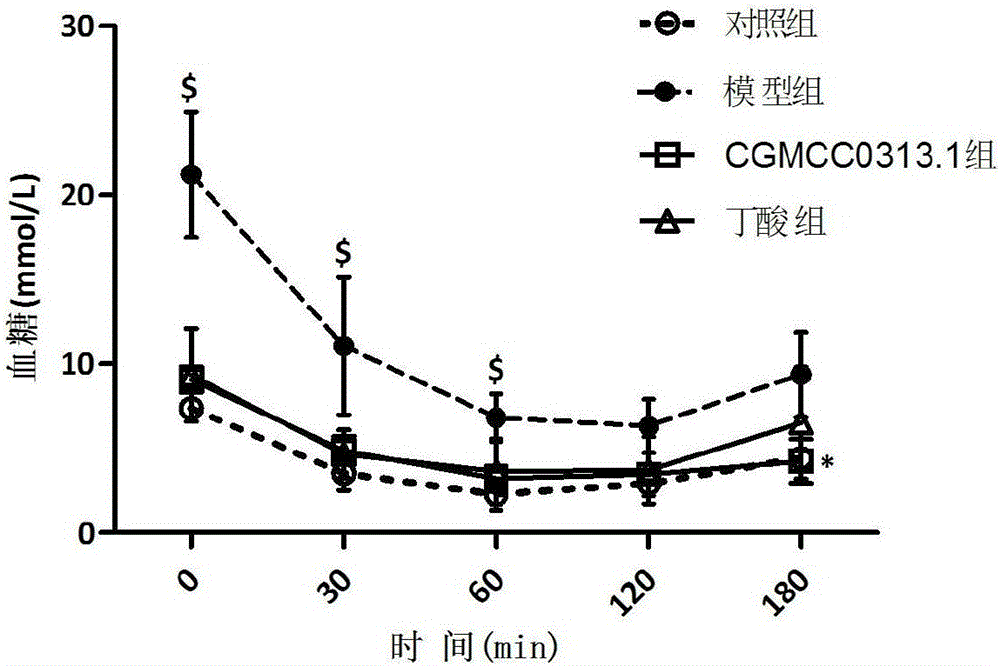
Application of clostridium butyrate in preparation for prevention and treatment or adjuvant therapy of hyperglycemia
ActiveCN105769928AChange compositionRecovery functionMetabolism disorderDigestive systemAcute hyperglycaemiaGlucagon-like peptide-1
The invention discloses an application of clostridium butyrate in a preparation for prevention and treatment or adjuvant therapy of hyperglycemia and belongs to the technical field of prevention and treatment of diabetes. According to the application, during the prevention and treatment or adjuvant therapy of the hyperglycemia, butyric acid is produced in an intestinal tract, meanwhile, florae of the intestinal tract are changed, signals are sent to an ileum, a liver, a pancreatic gland and a brain by a portal vein blood sugar sensor, the ileum is promoted to secrete (GLP-1) glucagon-like peptide-1, the gluconeogenesis of the liver is lowered, pancreatic beta cells are protected, the secretion of insulin of the pancreatic gland is promoted, and thus, the action of reducing blood sugar is exerted. The clostridium butyrate is a medicine which has been proven by clinical drug trials and can be directly taken orally.
Owner:QINGDAO EASTSEA PHARMA
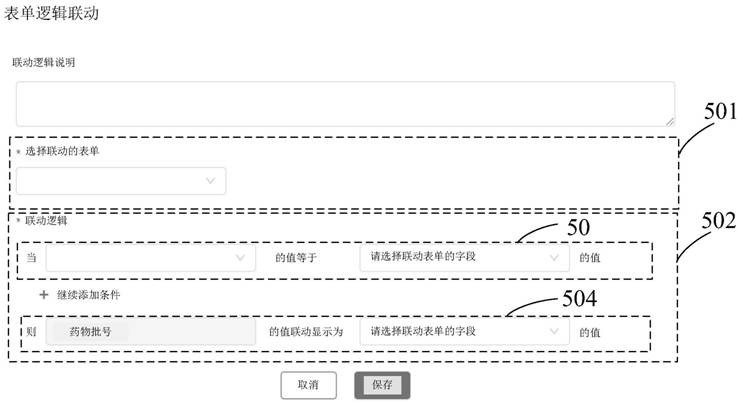
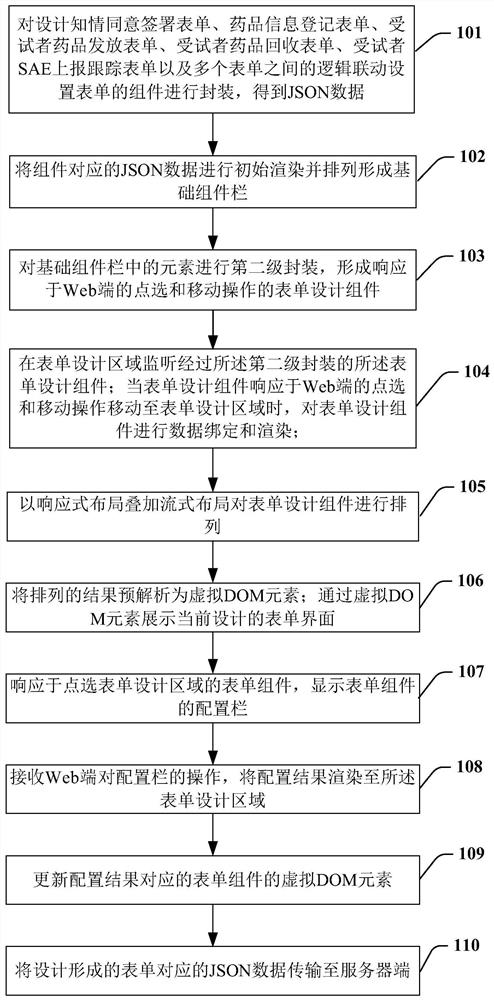
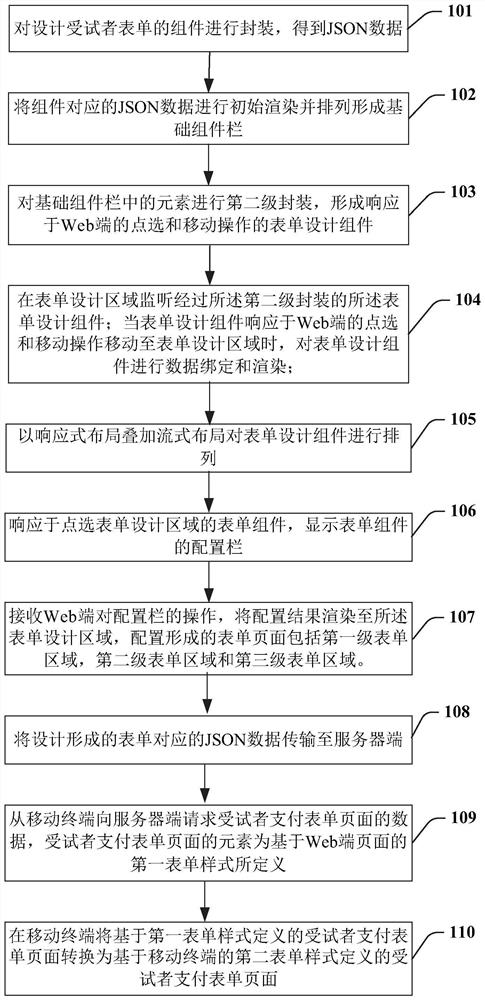
Implementation method and device of field management system for drug tests in clinical research
PendingCN111916163AGuaranteed uptimeQuick ViewNatural language data processingMedical reportsSoftware engineeringPharmaceutical drug
The invention provides an implementation method for a clinical research drug test field management system, and the field management system comprises an informed agreement signing form, a drug information registration form, a subject drug distribution and recovery form, a subject SAE reporting and tracking form, and a logic linkage setting form among a plurality of forms. The step of designing theplurality of forms comprises the following sub-steps: packaging components of the designed forms to obtain JSON data; carrying out initial rendering on the JSON data and arranging the JSON data to form a basic component column; forming a form design component responding to clicking and moving operations of the Web end; monitoring the form design component subjected to the second-level packaging inthe form design area; performing data binding and rendering on the form design component; arranging the form design components; pre-analyzing an arrangement result into a virtual DOM element; displaying a configuration column of the form component; receiving an operation on the configuration bar; and transmitting JSON data corresponding to the designed and formed form to a server side.
Owner:上海太美星云数字科技有限公司
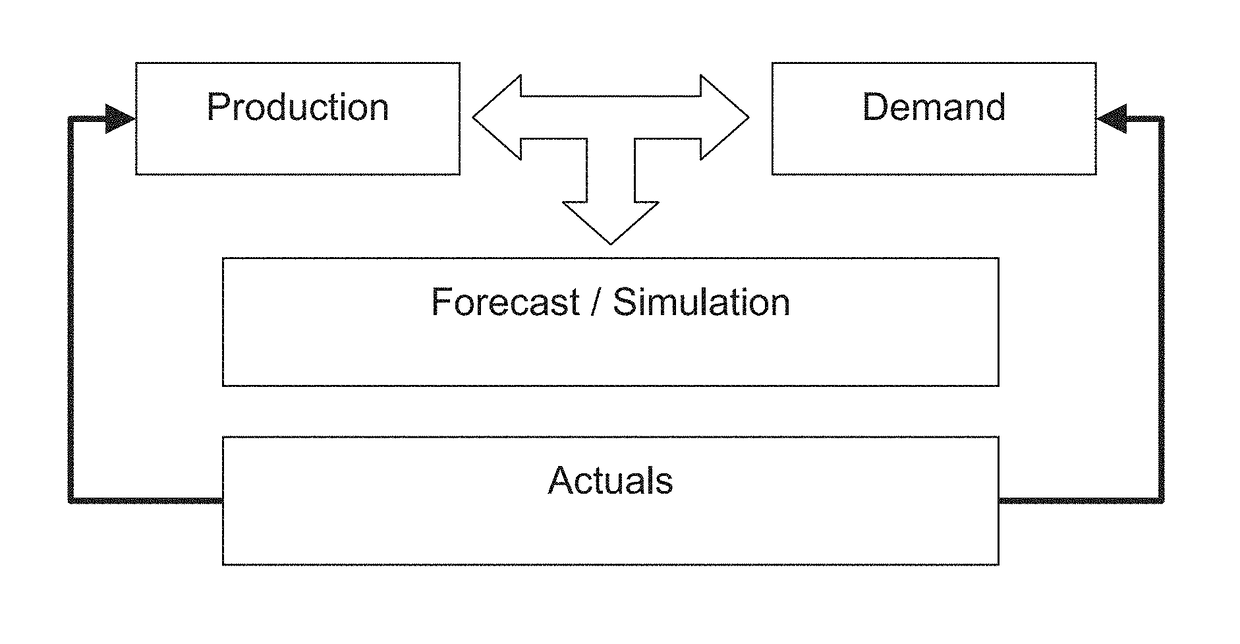
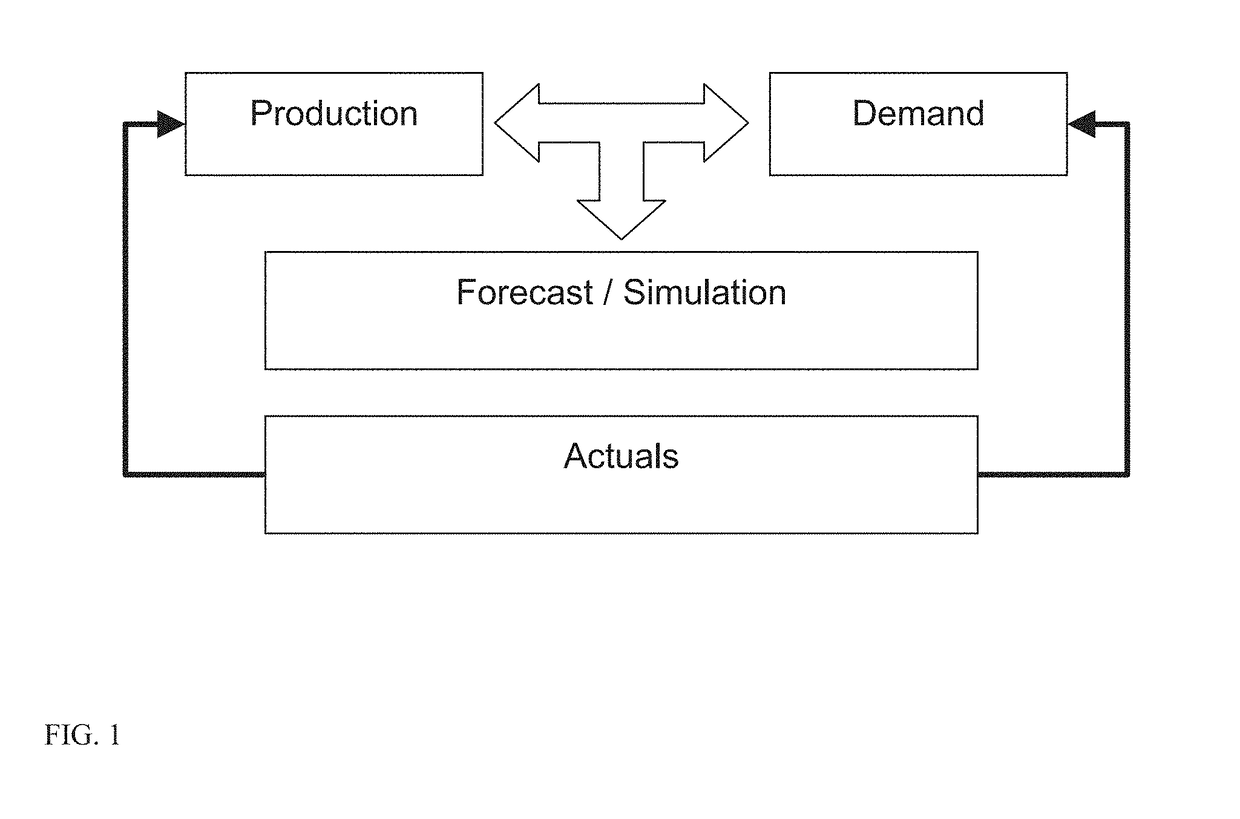
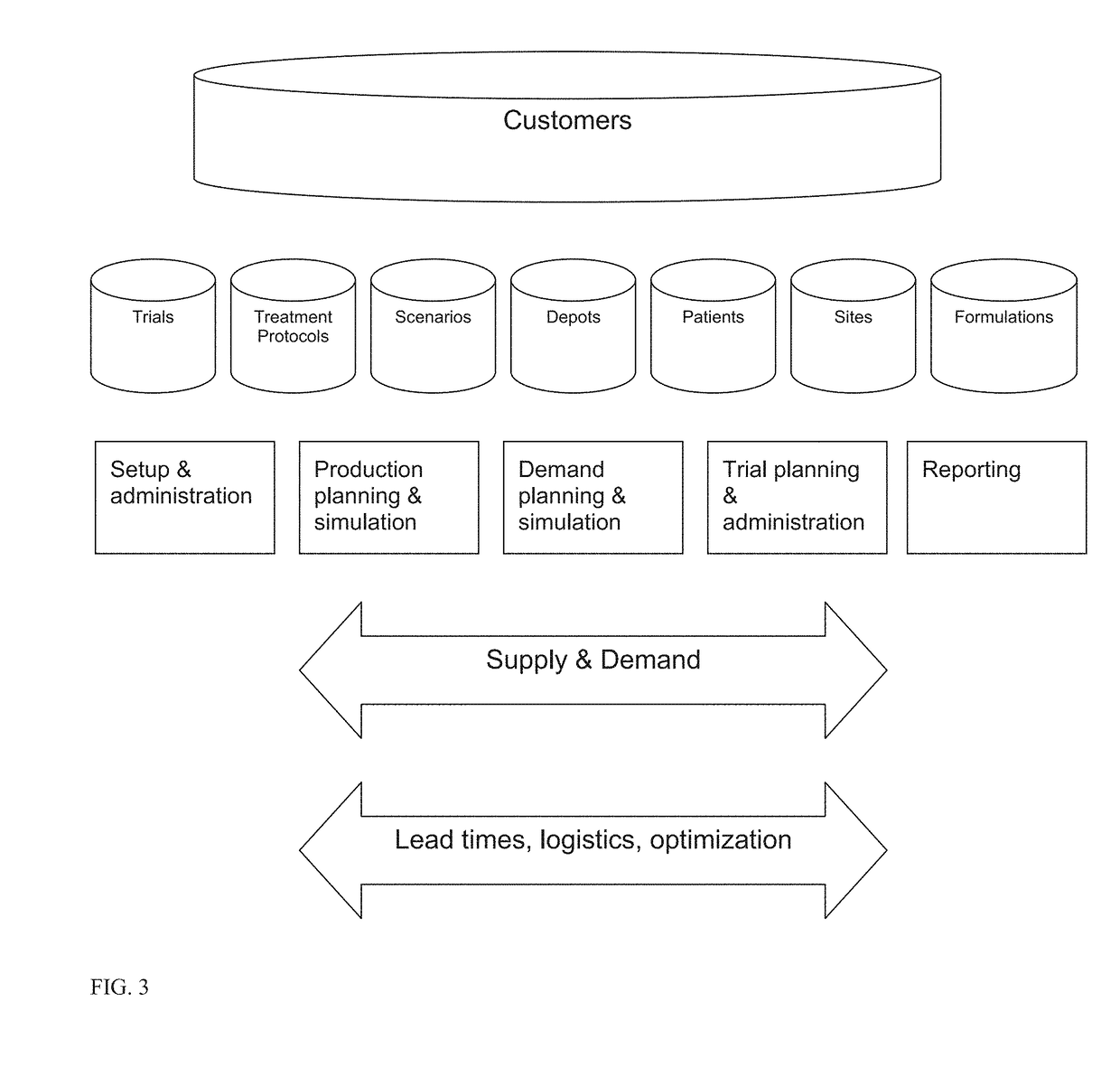
Clinical trial management and supply system and method
A system and method for planning, forecasting, administering, and managing the logistics of the supply chain for clinical trials for the products of pharmaceutical and biotechnology concerns includes providing functionality that eliminates the need to understand and directly manage the complexities of conducting a clinical trial by way of an integrated system of functional modules that provide capabilities in the areas of supply chain planning and forecasting (including logistics, budgeting, labeling, accountability, and destruction), recruitment planning and forecasting, utilization monitoring and tracking, and patient assignment and randomization, as well as simulating the conduct of such a clinical drug trial.
Owner:INNOVATIVE SUPPLY SOLUTIONS LLC
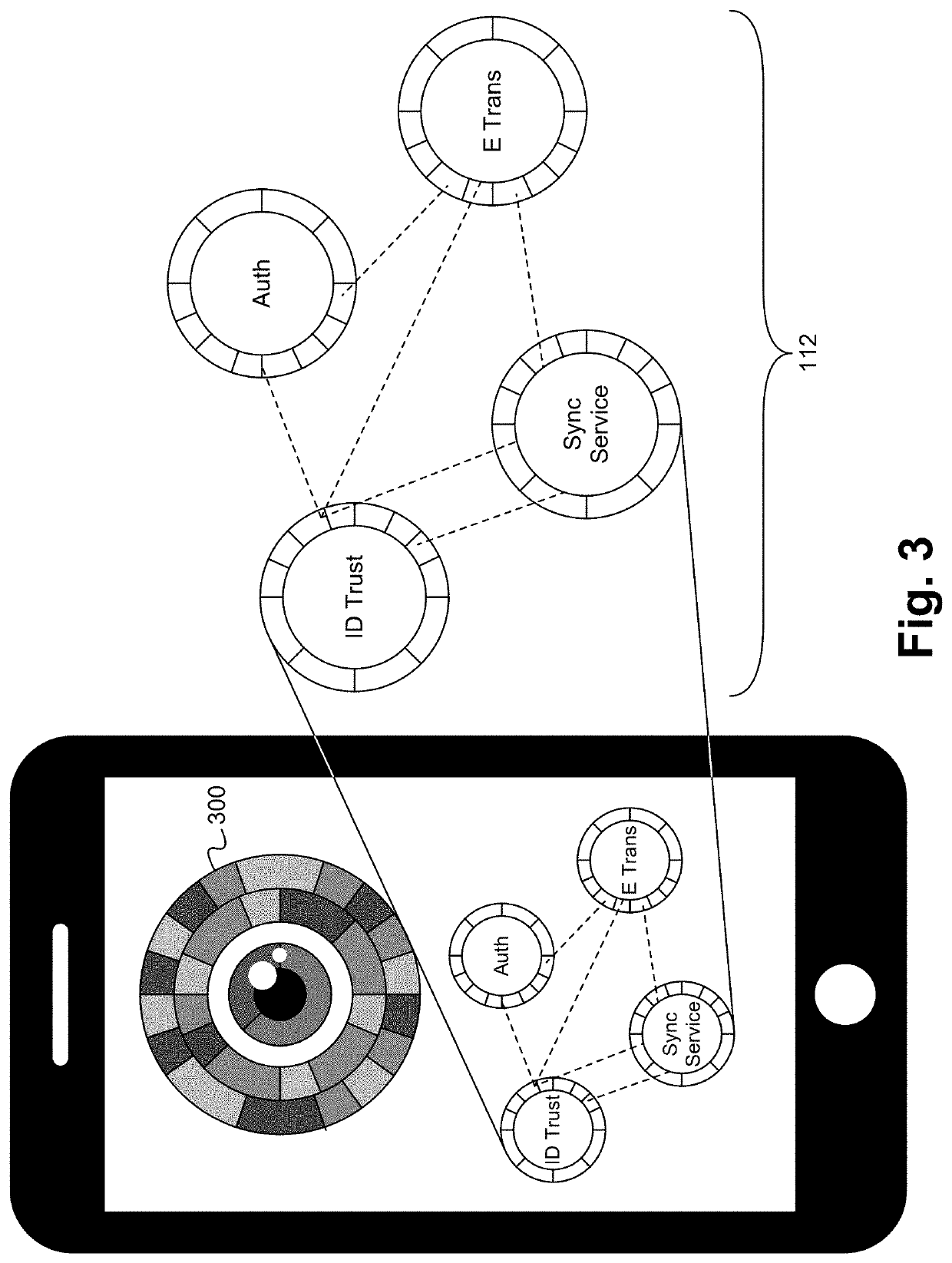
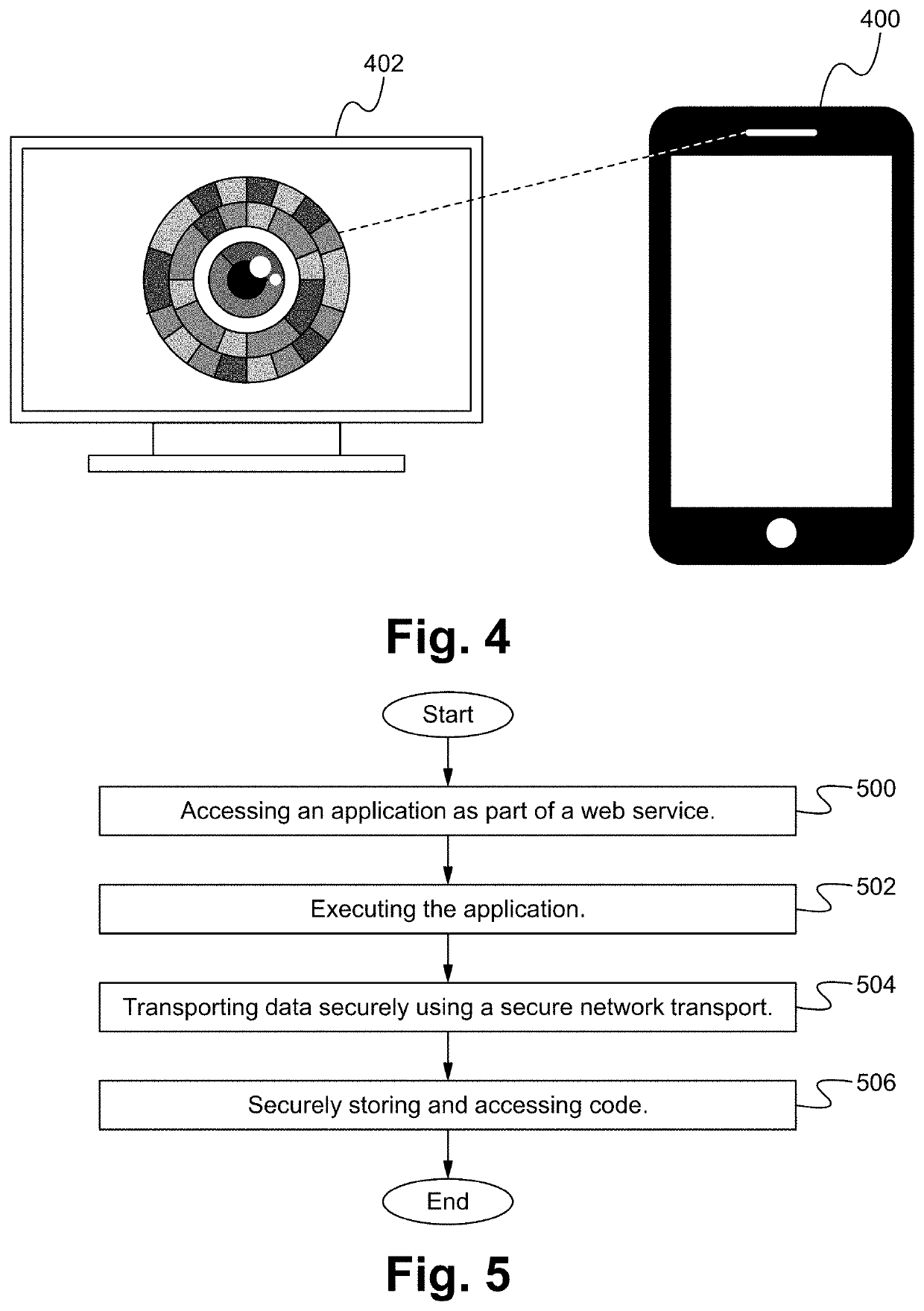
Clinical drug trial data enriching using activity and behavioral analytics captured with personal devices and apps
A security platform architecture is described herein. A user identity platform architecture which uses a multitude of biometric analytics to create an identity token unique to an individual human. This token is derived on biometric factors like human behaviors, motion analytics, human physical characteristics like facial patterns, voice recognition prints, usage of device patterns, user location actions and other human behaviors which can derive a token or be used as a dynamic password identifying the unique individual with high calculated confidence. Because of the dynamic nature and the many different factors, this method is extremely difficult to spoof or hack by malicious actors or malware software.
Owner:WINKK INC
Oral data collection device
Owner:HOOT MEDICAL ANALYTICS INC
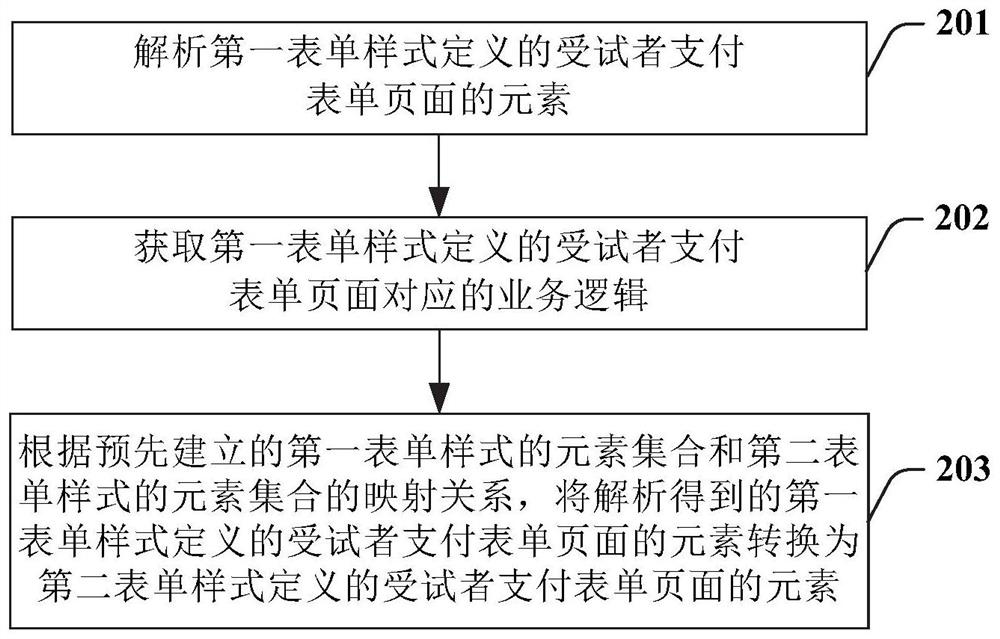
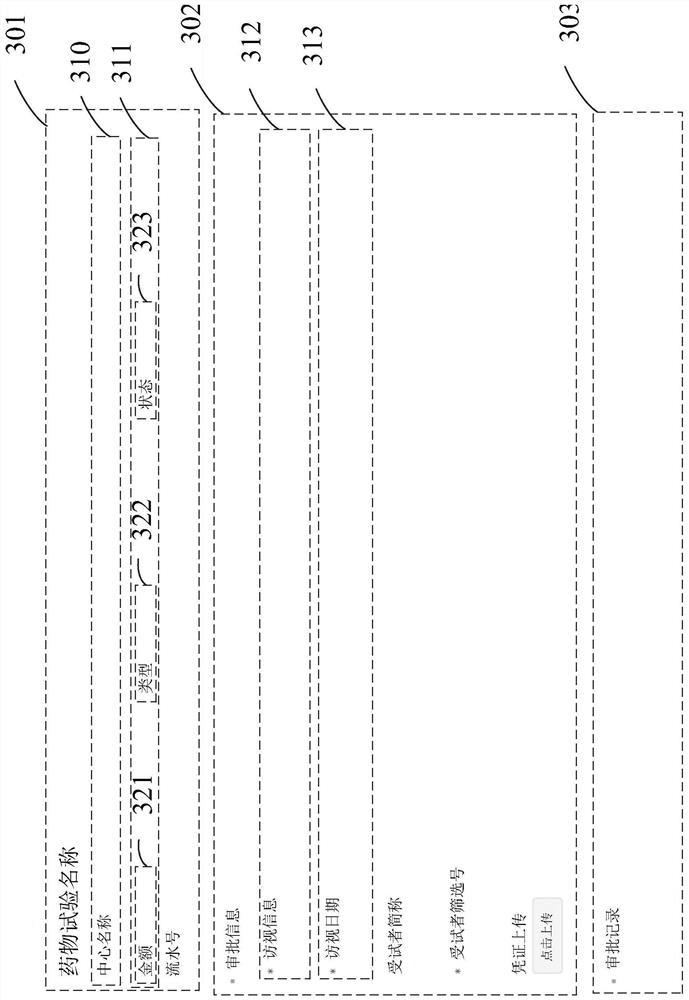
Implementation method and device of subject payment system for drug test in clinical research
PendingCN111931470AEasy to fillImprove settlement efficiencyNatural language data processingOffice automationClinical researchDrugs trials
The invention provides an implementation method of a subject payment system for a drug test in clinical research. The subject payment system comprises a Web terminal and a mobile terminal subject payment form page. The method comprises the following steps: packaging parts of a design form to obtain JSON data; forming a basic part column from the data corresponding to the part; performing second-level packaging on the elements in the basic part column to form a design part in response to clicking and moving operations; monitoring the part subjected to the second-level packaging in the form design area; performing data binding and rendering on the part; arranging the parts; displaying a configuration bar; receiving an operation of a Web end; transmitting data corresponding to the designed form to a server side; requesting data of a subject payment form page from the mobile terminal, wherein elements of the form page are defined by a first form style based on a Web terminal page; and converting the page defined by the first form style into a page defined by a second form style based on the mobile terminal at the mobile terminal.
Owner:上海太美星云数字科技有限公司
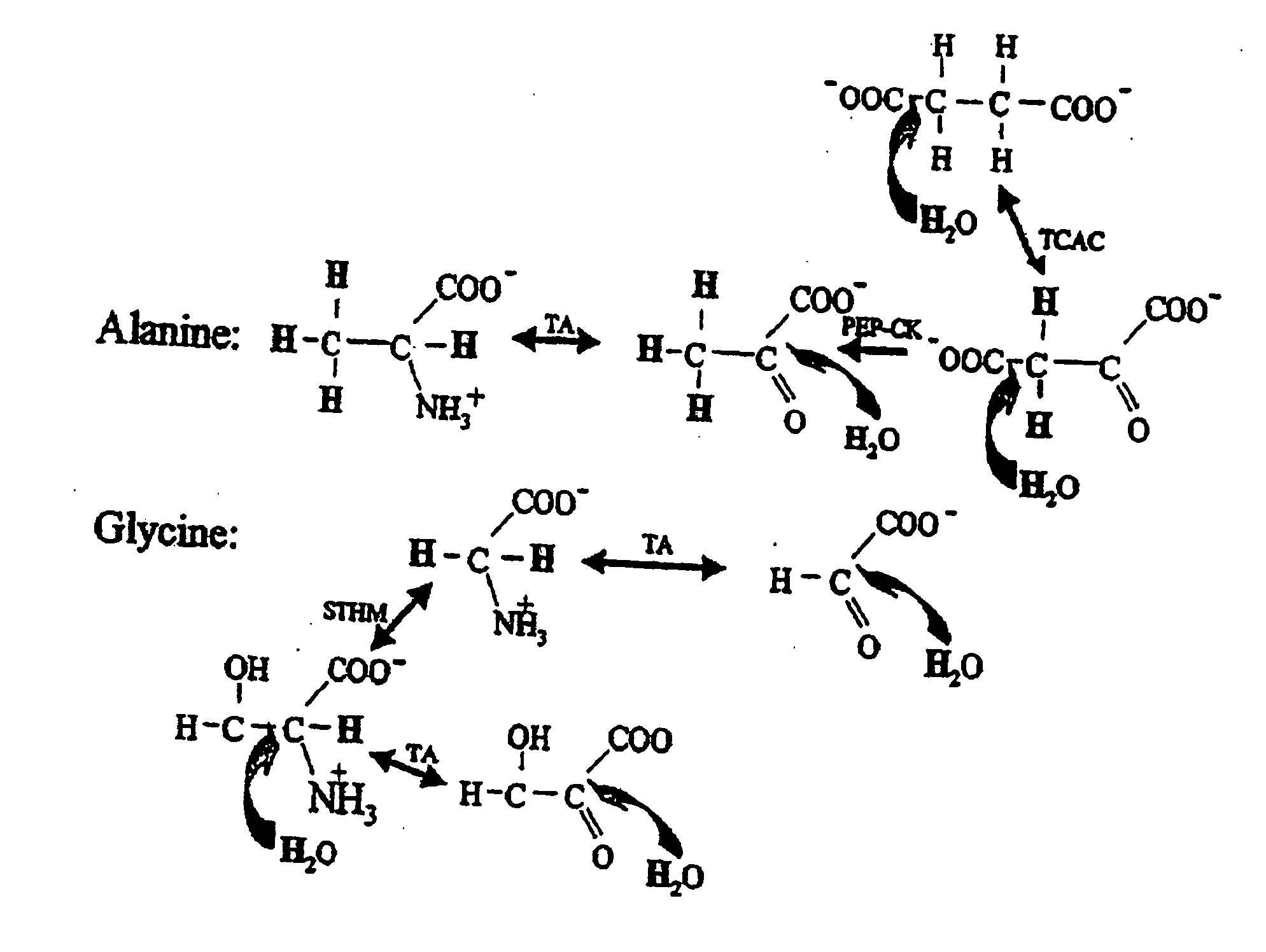
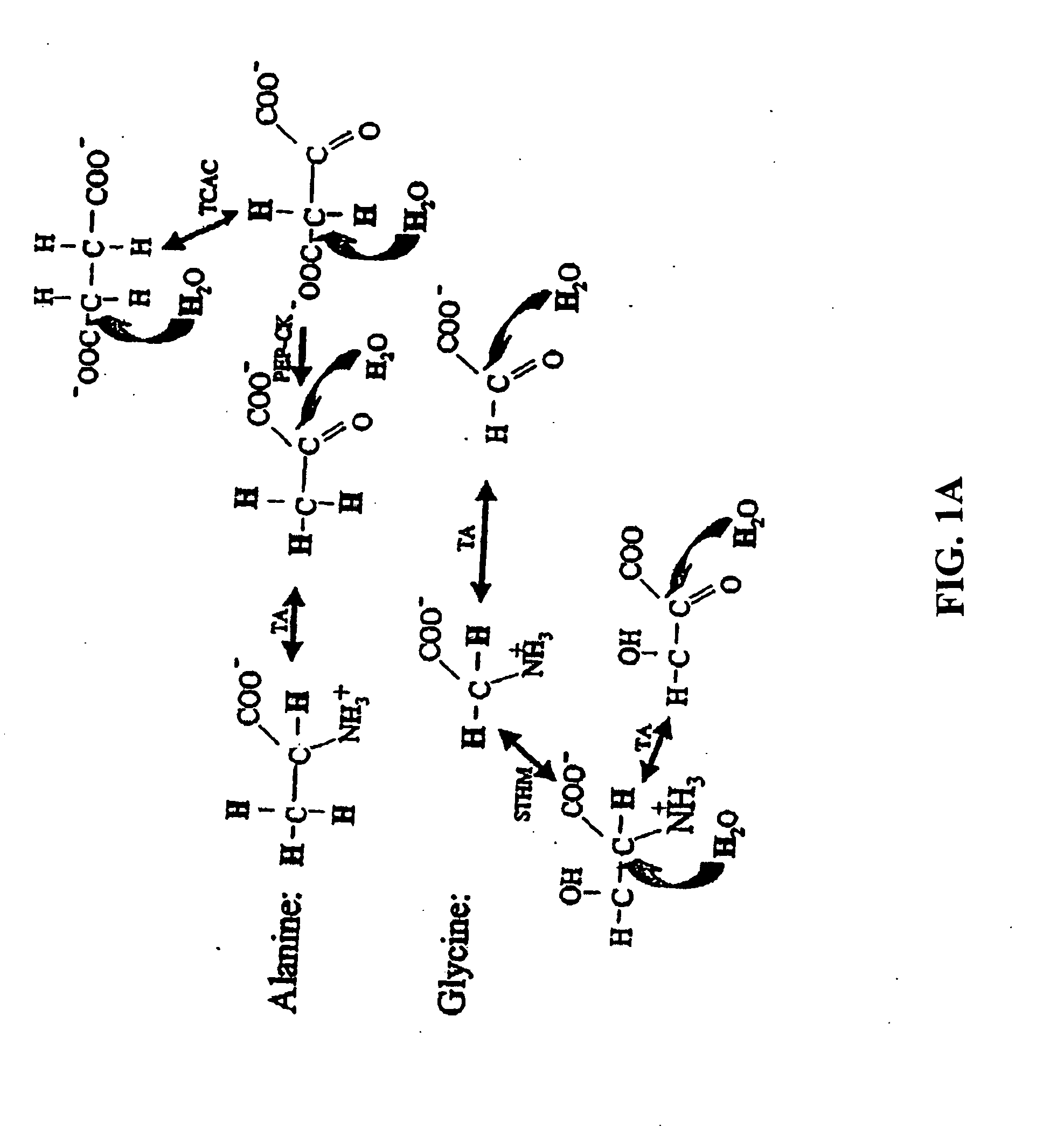
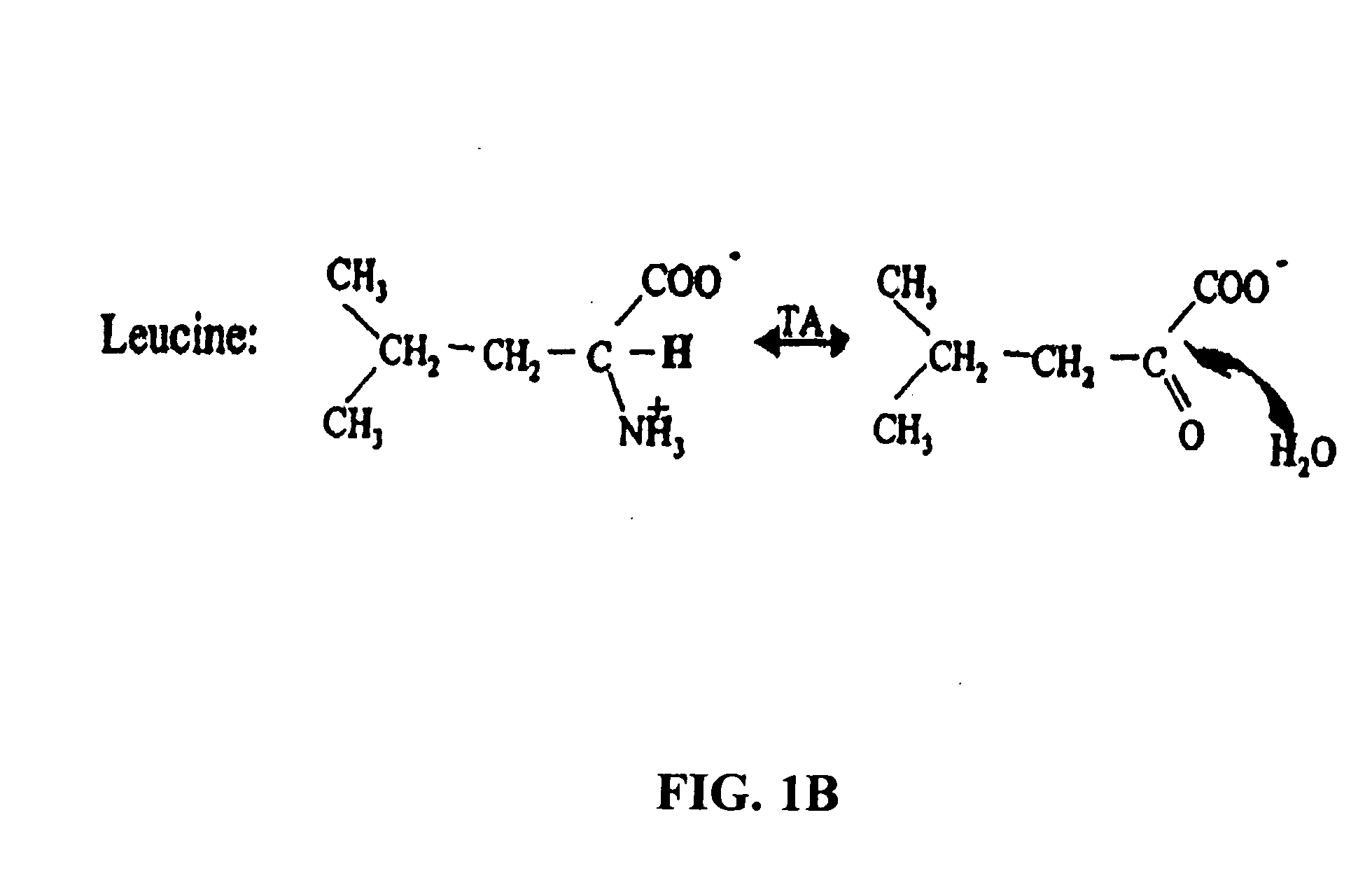
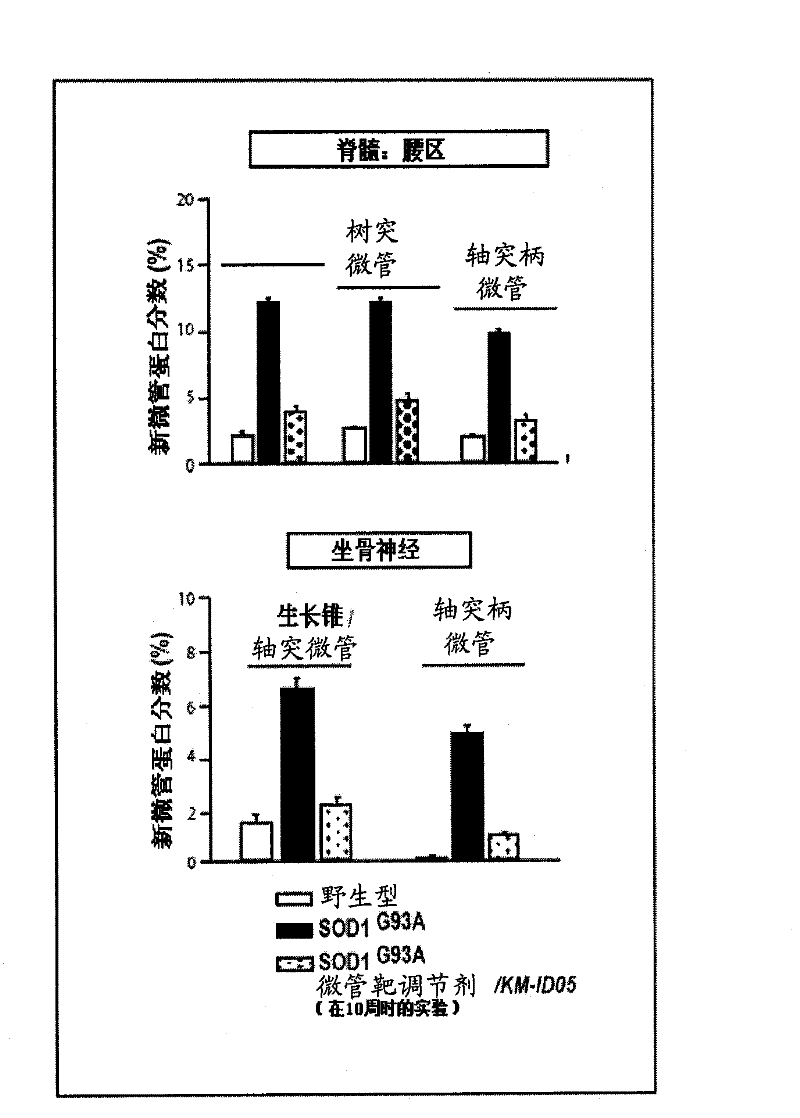
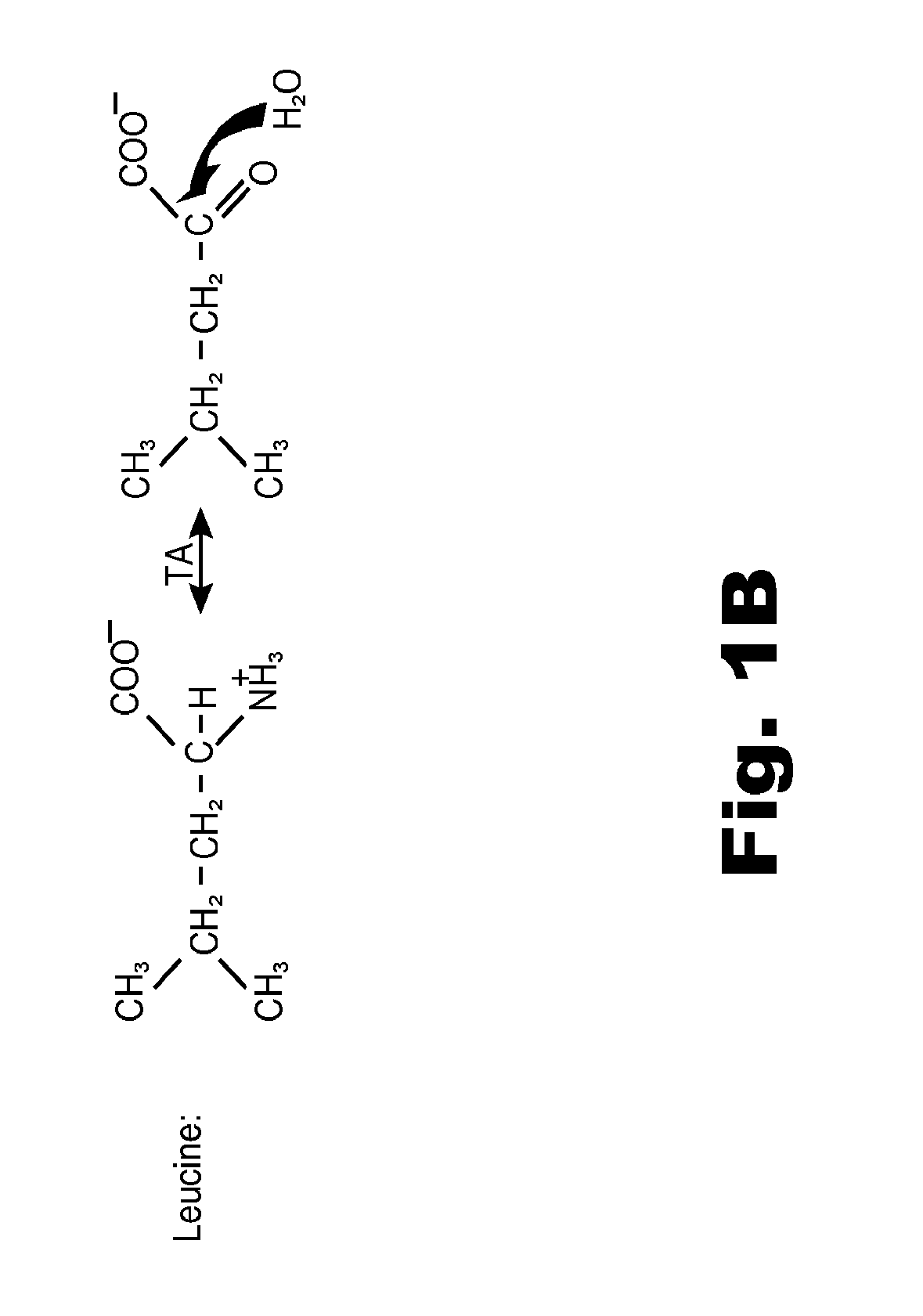
Compositions and methods of treatment using modulators of motoneuron diseases
The invention disclosed herein describes a novel therapeutic target for motoneuron diseases (altered dynamics of microtubules in neurons); a method for measuring the state of activity of this therapeutic target in subjects with established, incipient, or potential motoneuron disease; the discovery of drug agents that modulate neuronal microtubule dynamics in living subjects with motoneuron diseases; the discovery that administration of such agents, alone or in combinations, can provide marked neuroprotective therapy for living subjects with motoneuron diseases including delay in symptoms and prolongation of survival; and the discovery that monitoring of neuronal microtubule dynamics in subjects with motoneuron diseases, in response to therapeutic interventions, allows diagnostic monitoring for optimization of therapeutic regimen and strategy for individual subjects or for drug trials.
Owner:KINEMED
Compositions and methods of treatment using modulators of motoneuron diseases
InactiveCN102099687AOrganic active ingredientsCompound screeningTreatment strategyMediated transport
The invention disclosed herein describes a novel therapeutic target for motoneuron diseases (altered dynamics of microtubules in neurons); methods for measuring the state of activity of this therapeutic target in subjects with established, incipient, or potential motoneuron disease; the discovery of drug agents that modulate neuronal microtubule dynamics in living subjects with motoneuron diseases; the discovery that administration of such agents, alone or in combinations, can improve MT-mediated transport of ''cargo'' molecules along and through axons; the discovery that such modulation of altered microtubule dynamics and improvement in MT-transport of molecules along axons can provide marked neuroprotective therapy for living subjects with motoneuron diseases, including delay in symptoms and prolongation of survival; and the discovery that monitoring of neuronal microtubule dynamics in response to therapeutic interventions in subjects with motoneuron diseases,, allows diagnostic monitoring, to optimize therapeutic regimens and treatment strategies in individual subjects or in drug trials.
Owner:KINEMED
Clinical trial management and supply system and method
A system and method for planning, forecasting, administering, and managing the logistics of the supply chain for clinical trials for the products of pharmaceutical and biotechnology concerns includes providing functionality that eliminates the need to understand and directly manage the complexities of conducting a clinical trial by way of an integrated system of functional modules that provide capabilities in the areas of supply chain planning and forecasting (including logistics, budgeting, labeling, accountability, and destruction), recruitment planning and forecasting, utilization monitoring and tracking, and patient assignment and randomization, as well as simulating the conduct of such a clinical drug trial.
Owner:INNOVATIVE SUPPLY SOLUTIONS LLC
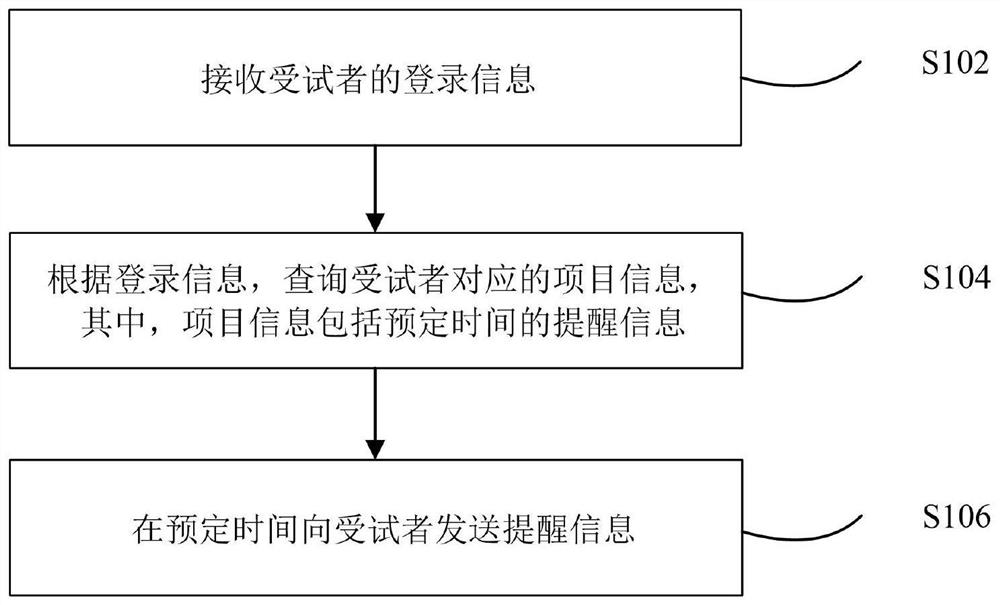
Information reminding method, system and device and storage medium
InactiveCN112185489AResolve to send AEResolve accuracyDrug and medicationsDrug referencesPharmacy medicineHospital information system
The invention relates to an information reminding method and system and computer equipment. The information reminding method comprises the steps: receiving the login information of a subject; according to the login information, searching item information corresponding to the subject, wherein the item information comprises reminding information of preset time; and sending the reminding informationto the subject in the preset time. According to the method and the device, the problem that an existing hospital information system cannot provide effective support for clinical drug tests and subjectmanagement, so that the subjects miss project reservation plans, and AE sending or data inaccuracy is caused is solved.
Owner:上海妙一生物科技有限公司
Compositions and methods of treatment using modulators of motoneuron diseases
The invention disclosed herein describes a novel therapeutic target for motoneuron diseases (altered dynamics of microtubules in neurons); methods for measuring the state of activity of this therapeutic target in subjects with established, incipient, or potential motoneuron disease; the discovery of drug agents that modulate neuronal microtubule dynamics in living subjects with motoneuron diseases; the discovery that administration of such agents, alone or in combinations, can improve MT-mediated transport of “synaptic vesicle cargo” molecules along and through axons; the discovery that such modulation of altered microtubule dynamics and improvement in MT-transport of molecules along axons can provide marked neuroprotective therapy for living subjects with motoneuron diseases, including delay in symptoms and prolongation of survival; and the discovery that monitoring of neuronal microtubule dynamics in response to therapeutic interventions in subjects with motoneuron diseases, allows diagnostic monitoring, to optimize therapeutic regimens and treatment strategies in individual subjects or in drug trials. The monitoring involves measuring isotope enrichment in secreted synaptic vesicle cargo molecules.
Owner:KINEMED
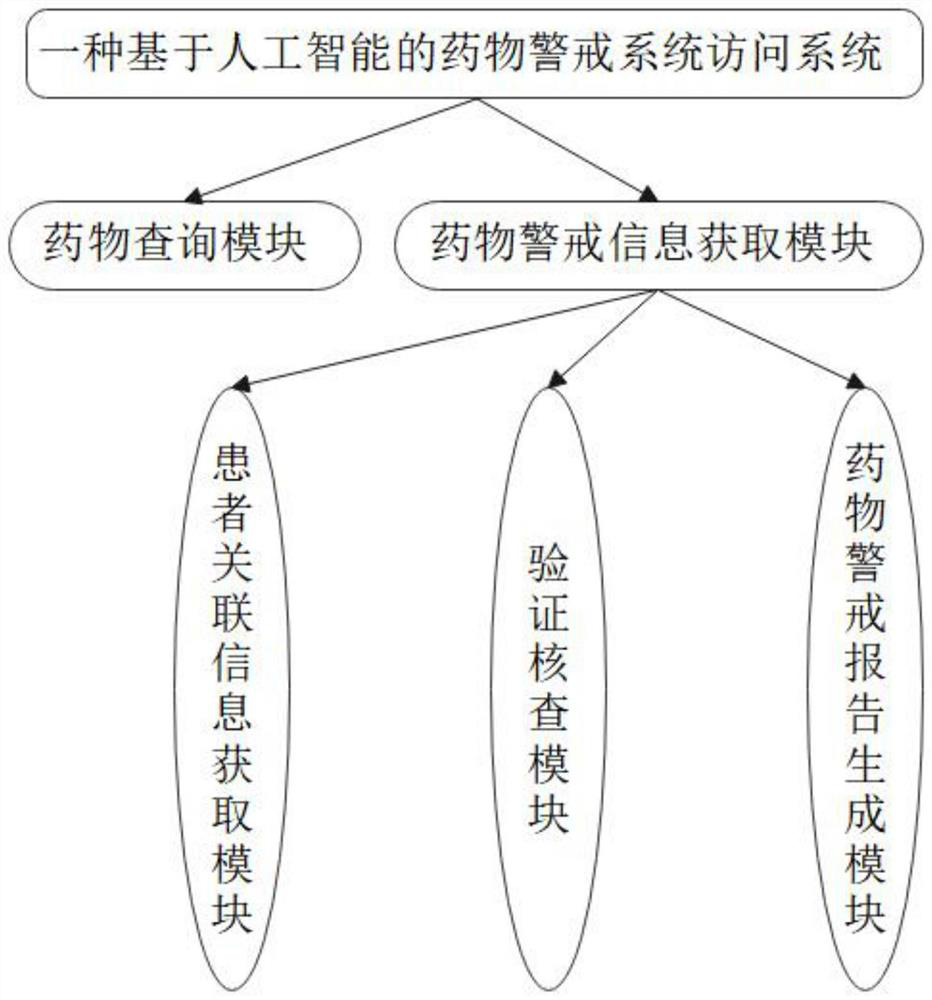
Drug alert system access system and method based on artificial intelligence
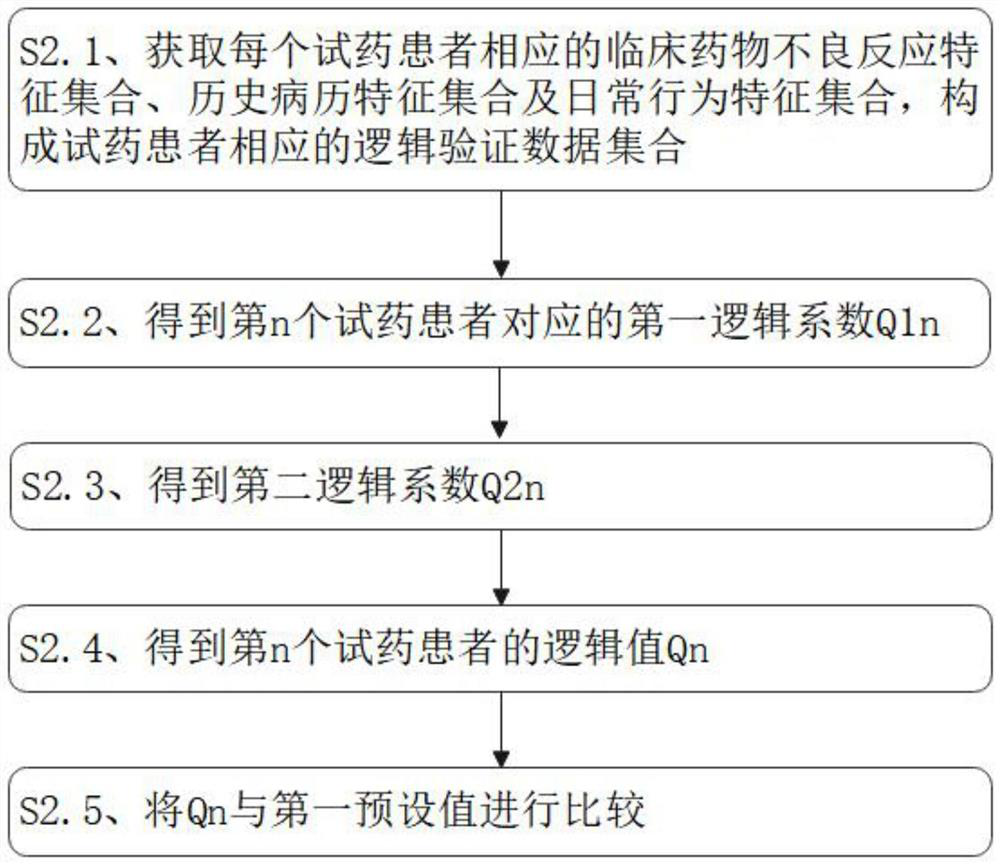
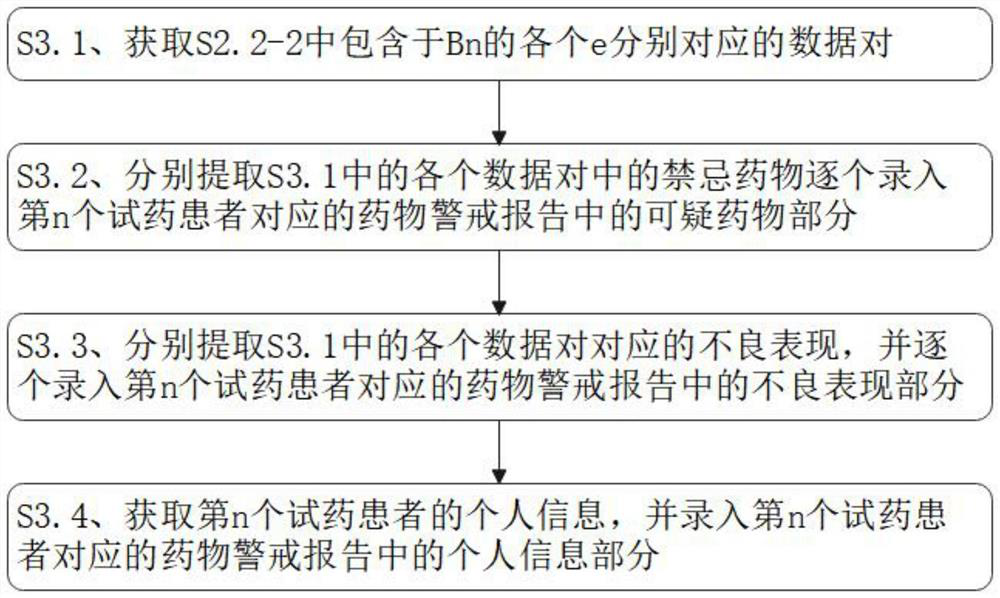
PendingCN114639458AImplement logical verificationRealize manual verificationDrug and medicationsDrug referencesPharmacy medicineMedicine
The invention discloses a drug alert system access system and method based on artificial intelligence, and the system comprises a drug query module which is used for querying the types of drugs to be accessed; the patient information acquisition module is used for acquiring various associated information of the drug trial patients aiming at the to-be-accessed drug types in the drug query module; the verification and verification module is used for carrying out logic verification on the associated information of the drug test patients of the to-be-accessed drug types obtained in the patient information acquisition module, judging whether suspicious information exists in the associated information or not, and carrying out manual verification on the suspicious information; and the medicine alert report generation module is used for summarizing the associated information of the medicine test patients of the to-be-accessed medicine types passing the verification and verification in the verification and verification module to obtain the medicine alert reports corresponding to the medicine test patients, and judging the priorities of the medicine alert reports according to the contents of the medicine alert reports.
Owner:HANGZHOU ZHUOJIAN INFORMATION TECH CO LTD
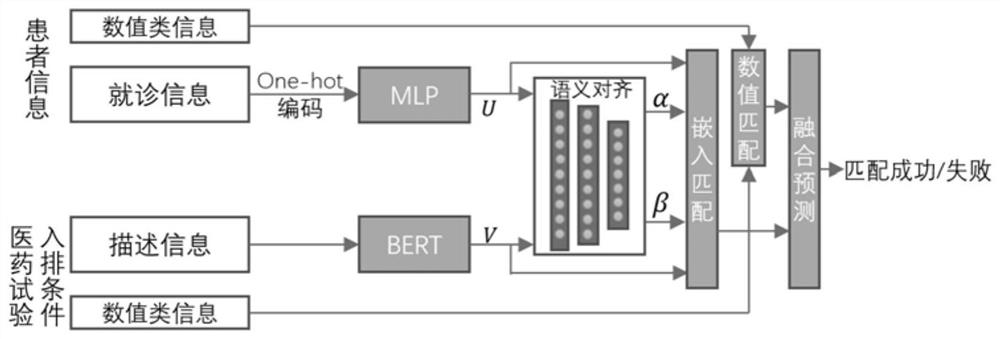
Clinical drug test patient matching method and device and computer equipment
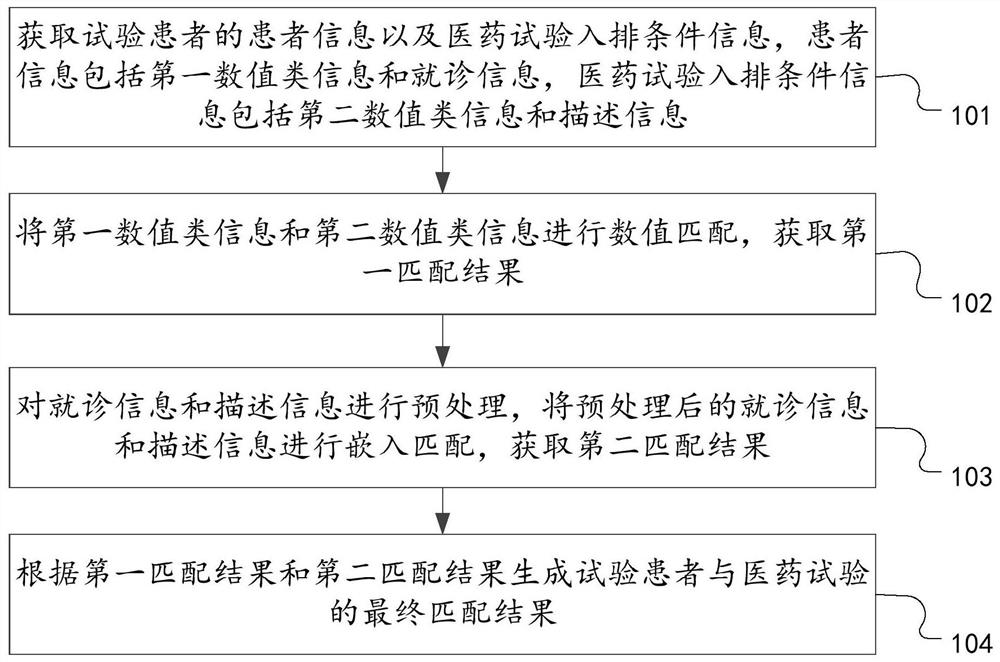
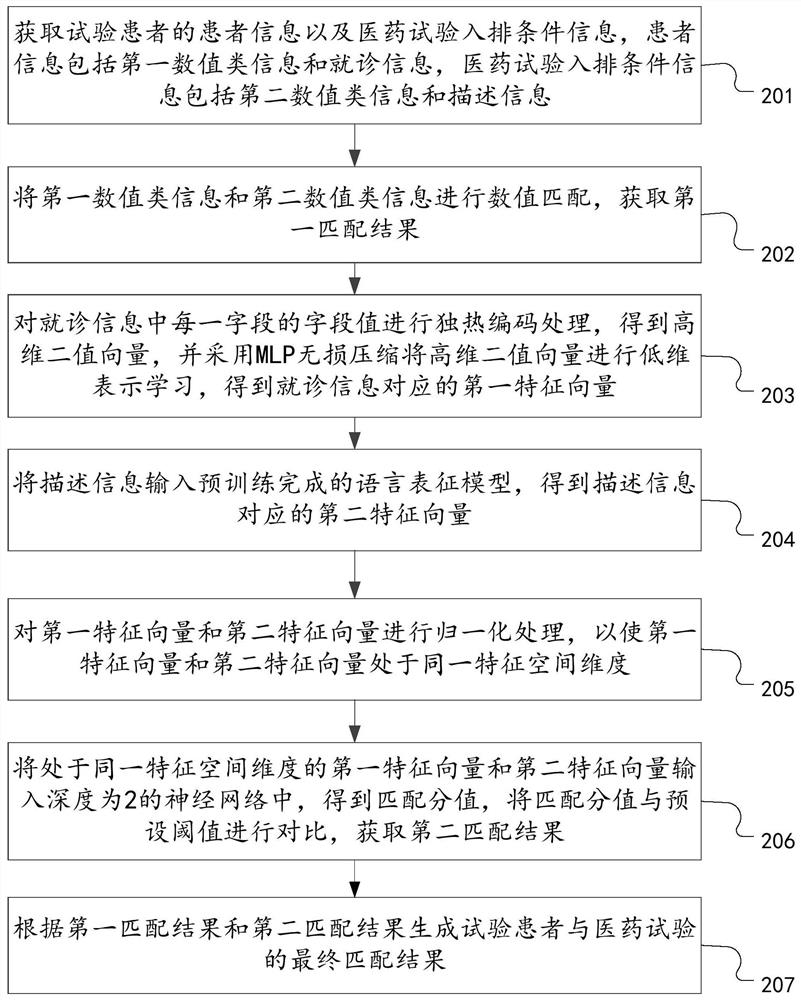
PendingCN114547238AAchieve precise matching effectTraditional Operations That Avoid Error AccumulationDrug referencesText database queryingSimulationPharmaceutical drug
The invention discloses a matching method and device for clinical drug test patients and computer equipment, relates to the technical field of computers, and can solve the technical problems that the workload is large, the efficiency is relatively low, and error accumulation and information loss are easily caused when the clinical drug test patients are matched at present. The method comprises the steps that patient information of a test patient and medical test queuing condition information are acquired, the patient information comprises first numerical value type information and doctor seeing information, and the medical test queuing condition information comprises second numerical value type information and description information; performing numerical value matching on the first numerical value type information and the second numerical value type information to obtain a first matching result; preprocessing the doctor-seeing information and the description information, and performing embedded matching on the preprocessed doctor-seeing information and description information to obtain a second matching result; and generating a final matching result of the test patient and the medicine test according to the first matching result and the second matching result.
Owner:北京水滴科技集团有限公司
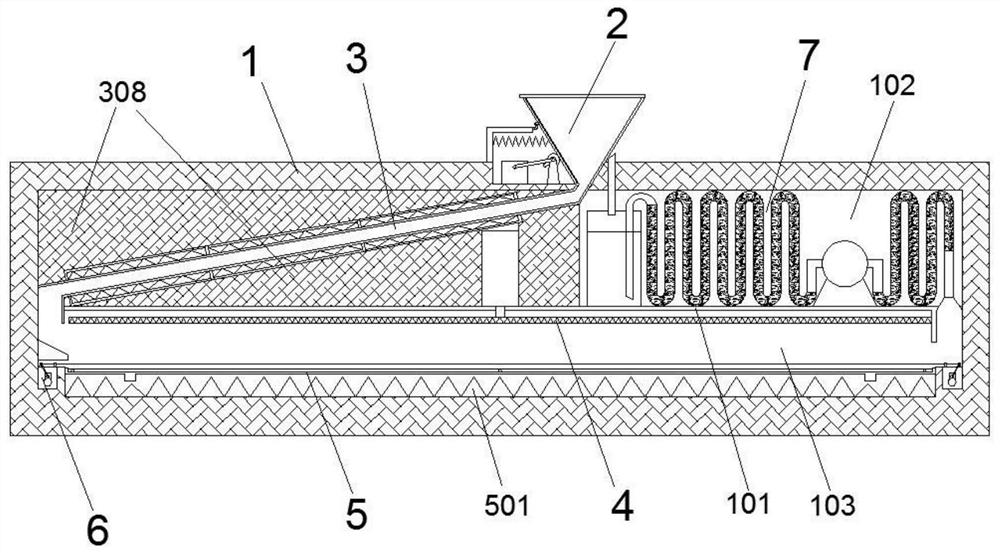
Hot-melt compression type blood collection tube recoverer for clinical drug tests
ActiveCN113478690APrevent re-flowAvoid breedingDispersed particle separationPlastic recyclingBlood Collection TubeWhole body
The invention relates to a hot-melt compression type blood collection tube recoverer for clinical drug tests. A partition plate is arranged in the middle of a shell and divides the shell into an upper cavity and a lower cavity, a preheating groove is formed in the upper cavity and communicates with a placement opening and the lower cavity, and therefore a blood collection tube is guided to fall onto a drawer type bearing mechanism arranged in the lower cavity; and the drawer type bearing mechanism has a heating function and is matched with a hot-pressing mechanism, and hot-melt compression of the blood collection tube in the drawer type bearing mechanism is achieved. The disposable blood collection tube can be preheated and then heated to be softened, the disposable blood collection tube is matched with an upper pressing plate for hot pressing to form a whole body for storage, the occupied area is saved, in the process, residual blood in the disposable blood collection tube can be evaporated to dryness, and bacterium breeding and peculiar smell generation are prevented; and more importantly, a plate damage type treatment mode is formed after hot-melt compression, and the disposable blood collection tube can be effectively prevented from flowing into the market again.
Owner:LUOYANG CENT HOSPITAL
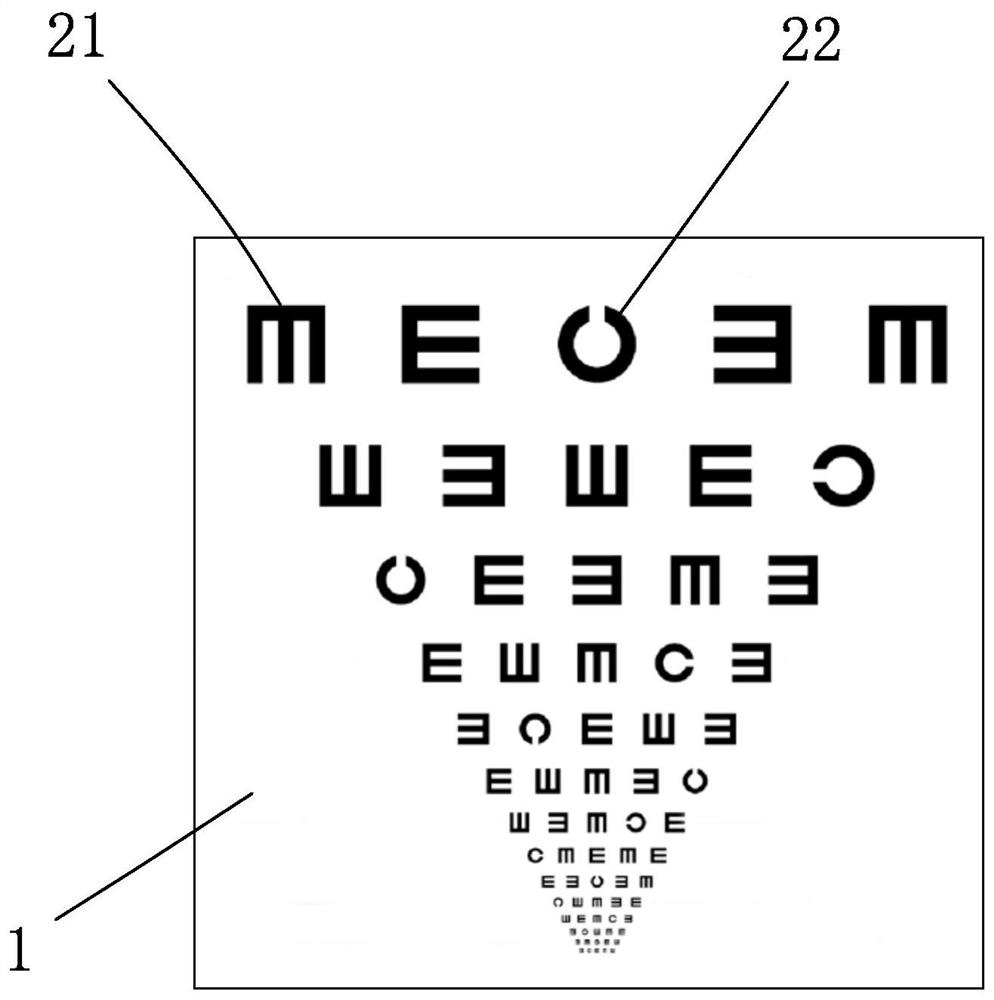
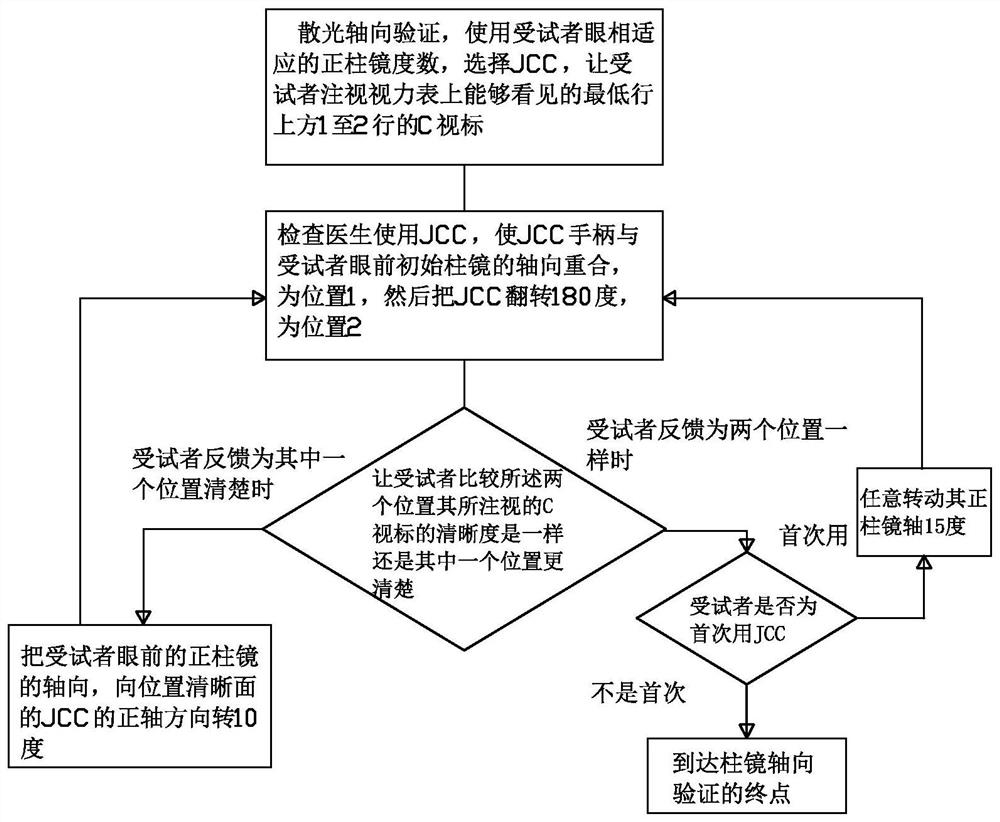
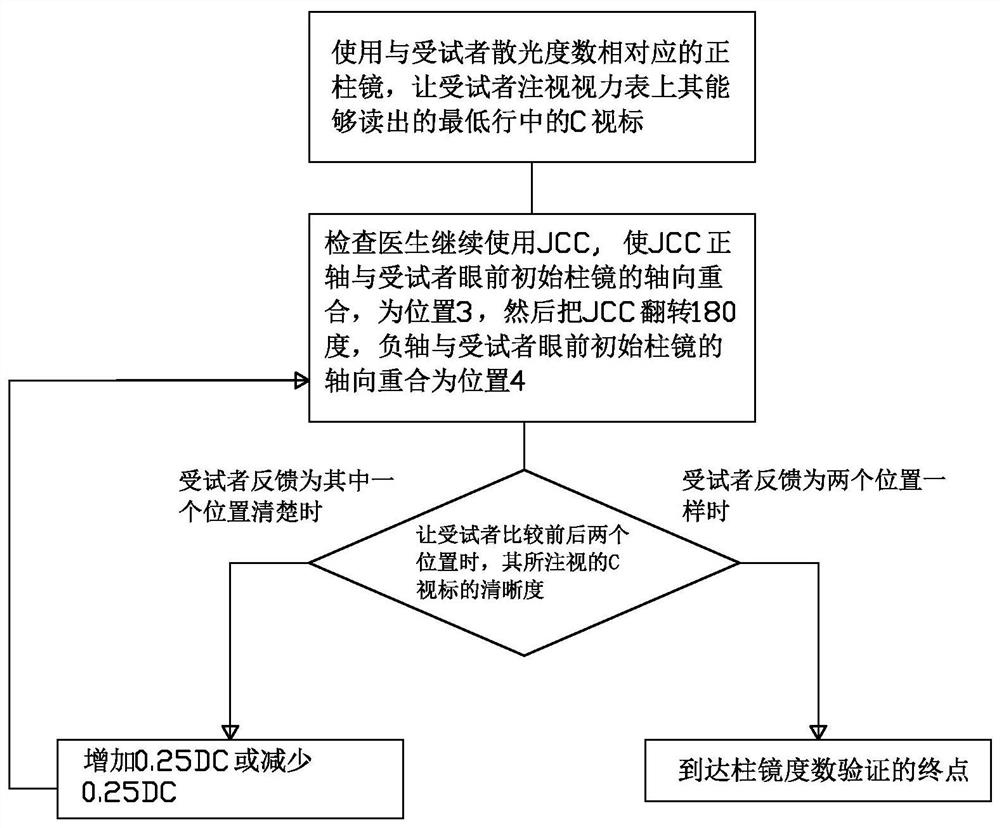
Visual chart suitable for clinical test and use method thereof
InactiveCN112754422AIn line with reading habitsHigh precisionEye diagnosticsClinical testsPharmaceutical drug
The invention discloses a visual chart suitable for a clinical test and a use method of the visual chart. The optotype size, the geometric increase rate, the line number, the optotype spacing and the line spacing of the visual chart all adopt international standards, and the innovation point is that each row of optotypes of an optometry chart (chart R) is composed of a C optotype with a gap and four rolling E optotypes. Each of the two optotypes has upward, downward, leftward and rightward orientations. The standardized operation specifications of the ETDRS visual chart and ETDRS optometry are gold standards of international clinical drug trials. On the basis of the rolling E optotypes commonly used by Chinese people, the combination of the two optotypes is provided in the newly designed optometry chart, namely, one C optotype exists in each row of rolling E optotypes, so that the requirements of identification and expression habits of Chinese people are better met during use, meanwhile, qualitative and quantitative measurement and verification of the axial direction and the degree of astigmatism of a subject can be performed, and the vision detection efficiency and accuracy can be improved.
Owner:温州佰思微医疗技术服务有限公司
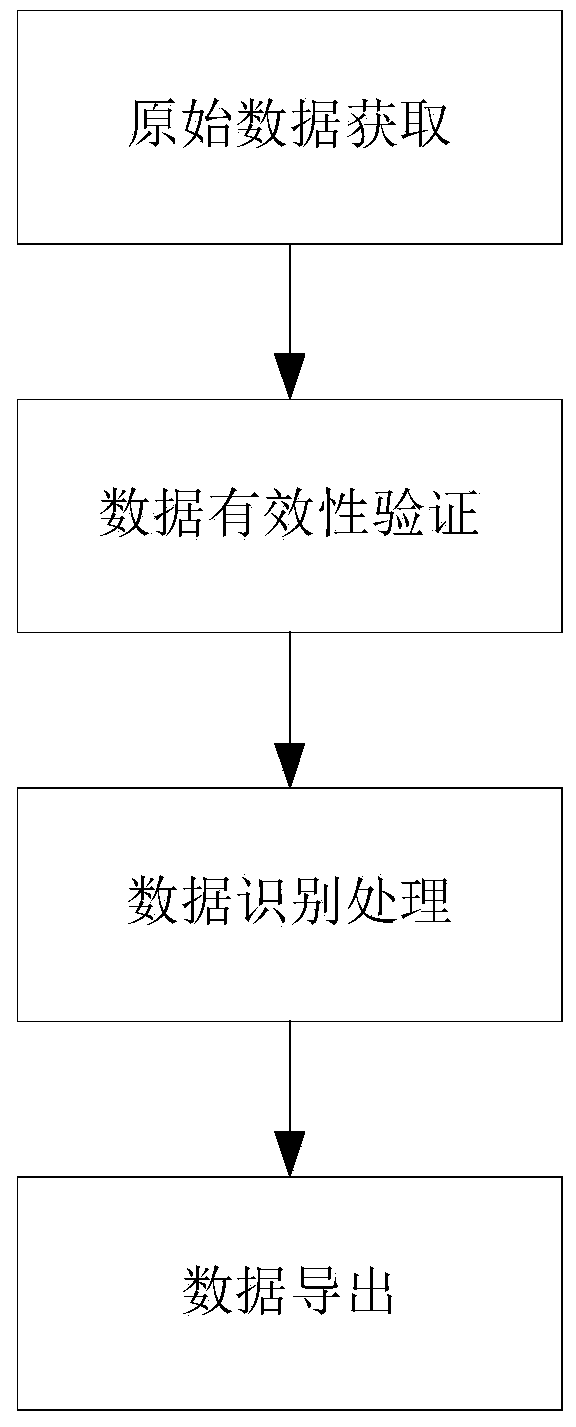
Visual identification screening method for adverse events of drug test
ActiveCN111427948AImprove screening efficiencyImprove efficiencyVisual data miningStructured data browsingPharmaceutical drugTest object
The invention discloses a visual identification screening method for adverse events of a drug test. The method comprises the following steps: acquiring baseline period data and test period data of each test object from an EDC database; performing validity verification on the acquired baseline period data and the test period data, and screening out a test object to be identified; performing identification processing on the baseline period data and the test period data of each to-be-identified test object, and outputting visual data convenient to identify; and popping up and displaying the visual data is popped up and displayed, so that visual review and check are facilitated. According to the visual identification screening method, complete and effective test objects of baseline period dataand test period data can be screened out, so that manual integrity and effectiveness check is avoided, and the screening efficiency and accuracy of adverse events can be effectively improved; and meanwhile, the test object data after validity verification can be identified and sorted, so that the visual examination of personnel is facilitated, and the discrimination efficiency is further improved.
Owner:JIANGSU LEEWAY BIOLOGICAL TECH
Oral data collection device
ActiveUS10945665B1Measurement stabilityPredict rate of changeHumidity sensorsOthrodonticsAnatomical structuresMouthguard
The present disclosure provides an oral data collection device comprising a mouthguard and at least two sensors, a medical analytics system comprising the device, and methods of using the device and system to provide a personalized treatment protocol, stage a health condition, measure a response to therapy, phenotype for selection to participate in drug trials, measure stability of an anatomical structure, or predict a rate of change of a health condition in a subject in need thereof.
Owner:HOOT MEDICAL ANALYTICS INC
Compositions and methods of treatment using modulators of motoneuron diseases
The invention disclosed herein describes a novel therapeutic target for motoneuron diseases (altered dynamics of microtubules in neurons); a method for measuring the state of activity of this therapeutic target in subjects with established, incipient, or potential motoneuron disease; the discovery of drug agents that modulate neuronal microtubule dynamics in living subjects with motoneuron diseases; the discovery that administration of such agents, alone or in combinations, can provide marked neuroprotective therapy for living subjects with motoneuron diseases including delay in symptoms and prolongation of survival; and the discovery that monitoring of neuronal microtubule dynamics in subjects with motoneuron diseases, in response to therapeutic interventions, allows diagnostic monitoring for optimization of therapeutic regimen and strategy for individual subjects or for drug trials.
Owner:KINEMED
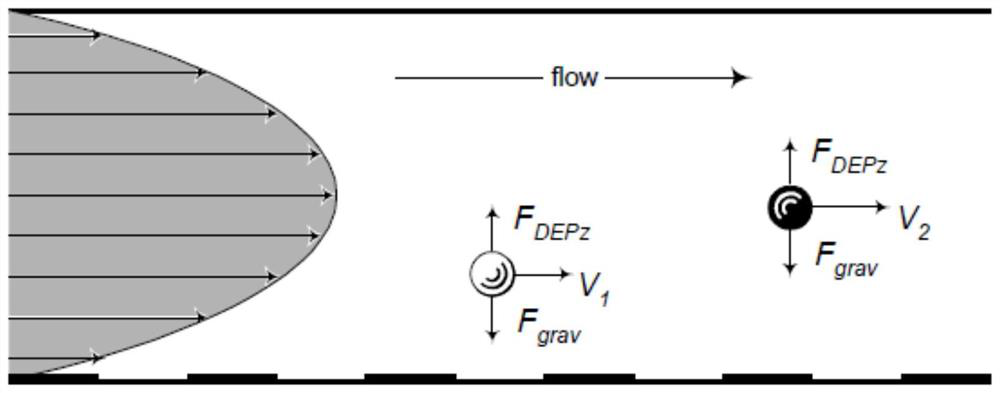
A method for separating and enriching platelets, a drug testing method for platelet-acting drugs, and a drug testing chip
ActiveCN108918797BEasy to separateEfficient separationMaterial analysis by electric/magnetic meansTesting medicinal preparationsDielectrophoretic forceBlood specimen
The invention discloses a platelet separation and enrichment method, a platelet effect drug trial method and a platelet effect drug trial chip, and relates to the field of platelet detection. The platelet separation and enrichment method is as follows: allowing a blood sample to flow in a pipe, applying a dielectrophoretic force; adding an inducer to platelets; allowing the mixture to flow in thepipe, applying a dielectrophoretic force, and detecting amounts of aggregated platelets and platelets. The platelet separation and enrichment method can quickly and effectively separate the plateletsfrom the blood sample. The platelet effect drug trial method is as follows: adding a platelet effect drug to the mixture; allowing the mixture after the action of the drug to flow in the pipe, applying a dielectrophoretic force, and detecting the amounts of aggregated platelets and platelets, and the platelet effect drug trial method can quickly detect changes in aggregation behaviors of the platelets after the action of the drug. The platelet effect drug trial chip has a simple structure and a low cost, and can quickly perform a platelet effect drug trial.
Owner:WUHAN YZY MEDICAL SCI & TECH
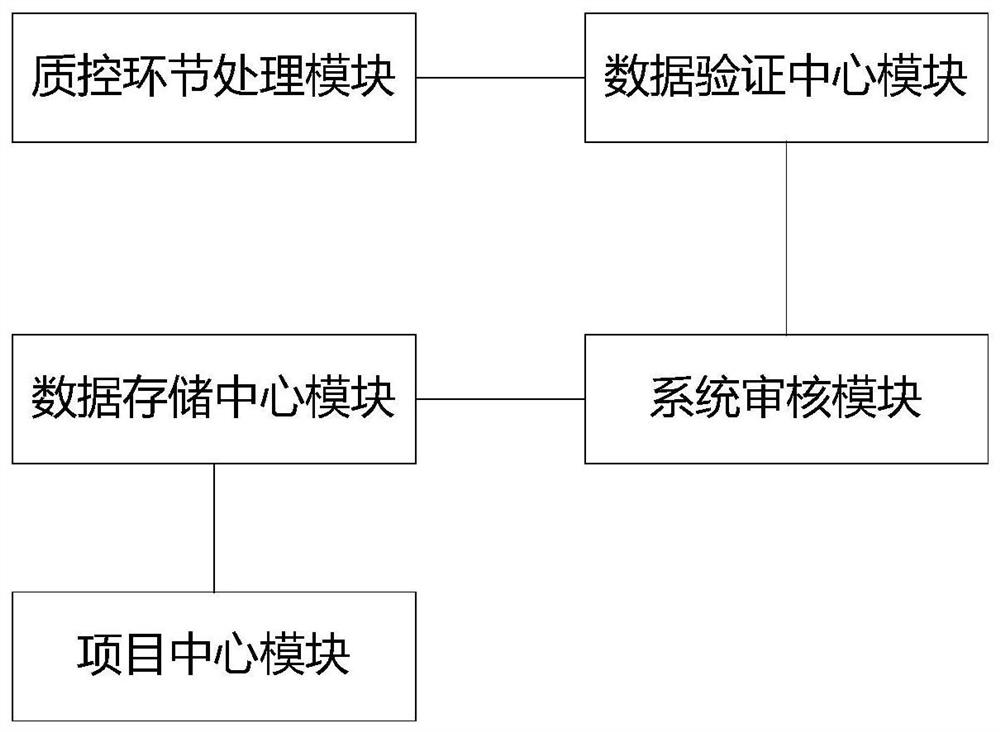
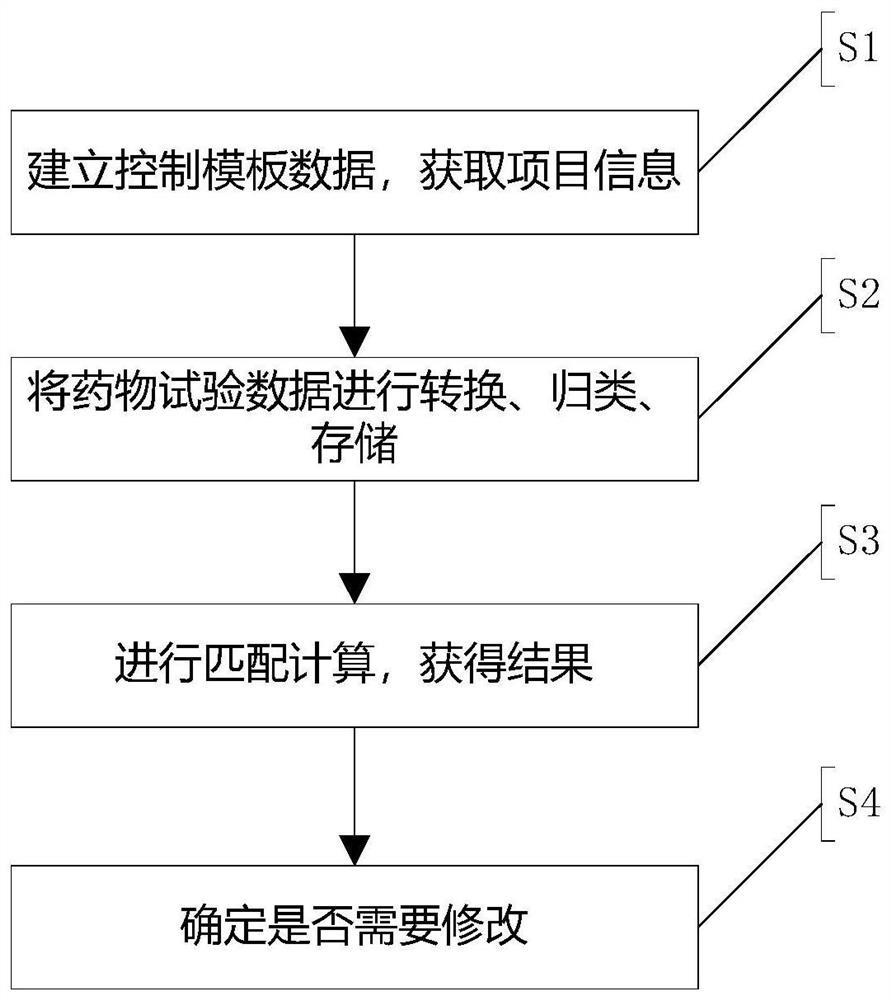
Drug test data control method and system
PendingCN113450928AReduce error rateImprove carding efficiencyDrug referencesData transformationSource Data Verification
The invention discloses a drug test data control method and system, and the system comprises: a quality control link processing module which is used for inputting control template data based on a quality control point of a drug clinical test; a data verification center module which is used for receiving the control template data of the quality control link processing module and converting non-standard data in the control template data into uniform structural data; a project center module which is used for summarizing clinical test project information and serves as a unified output port of the clinical test project information; a data storage center module which is used for storing effective data in clinical test items; and a system auditing module which is used for performing calculation based on the effective data of the clinical test project to obtain a project quality control content item. According to the scheme, full-process monitoring and processing of the drug test data are achieved, the error rate in the processes of data recording, screening and the like is effectively reduced, the carding and storage efficiency of the data is greatly improved, and the quality and effectiveness of basic data are improved.
Owner:BEIJING ANZHEN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
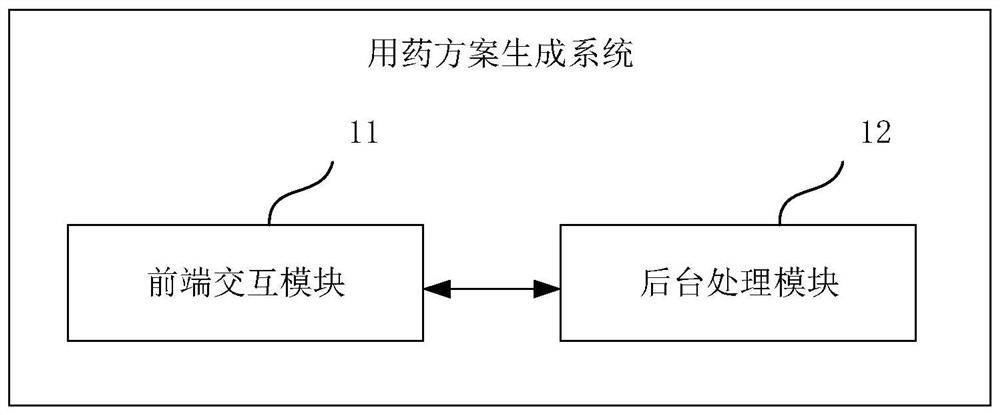
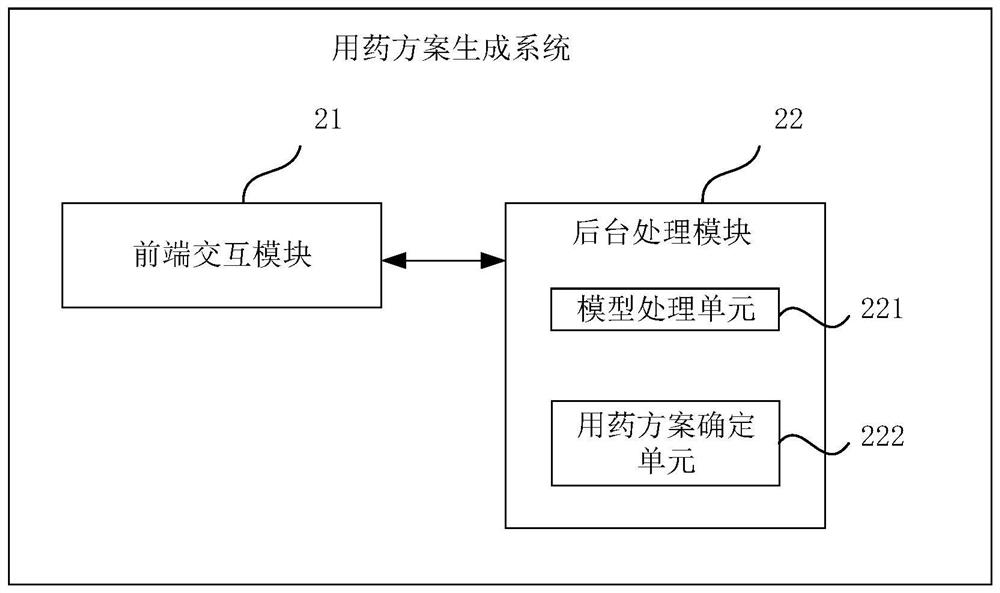
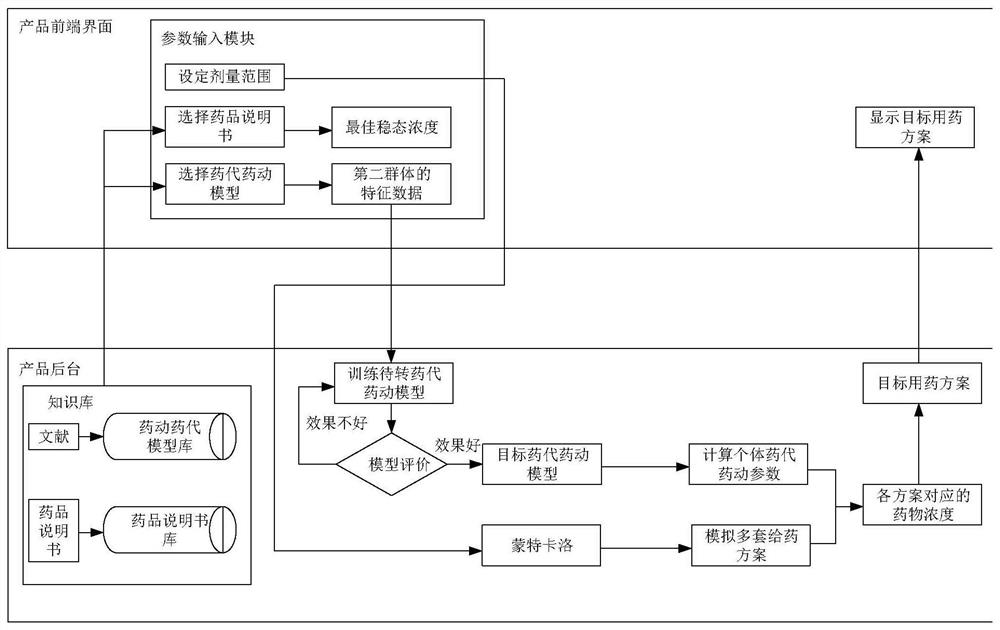
Medication scheme generation system and method
PendingCN113921148AImprove production efficiencyEnsure safety and effectivenessMedical communicationDrug referencesDrug utilisationMedication information
The embodiment of the invention discloses a medication scheme generation system and method. The system comprises a front-end interaction module and a background processing module, through the front-end interaction module, a model selection operation, a specification selection operation and a dose setting operation executed by a user on a displayed medication scheme generation interface are obtained, through the background processing module, a to-be-transferred pharmacokinetic model selected by the user in the pharmacokinetic model library, a target drug specification selected by the user in the drug specification library and dose range information are determined respectively, a target medication scheme is generated based on the to-be-transferred pharmacokinetic model, the target drug specification and the dose range information and fed back to the front-end interaction module, therefore, the front-end interaction module displays the target medication scheme on the medication scheme generation interface, the medication information of other people is determined by using the existing data, the medication test on other people is avoided, and the efficiency of generating the medication scheme is improved.
Owner:联仁健康医疗大数据科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com