Visual identification screening method for adverse events of drug test
A technology of adverse events and screening methods, applied in electronic clinical trials, visual data mining, office automation, etc., can solve problems such as inability to meet data management requirements, time-consuming and laborious, and affect conclusions, so as to facilitate visual review and improve screening efficiency , Improve screening efficiency and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
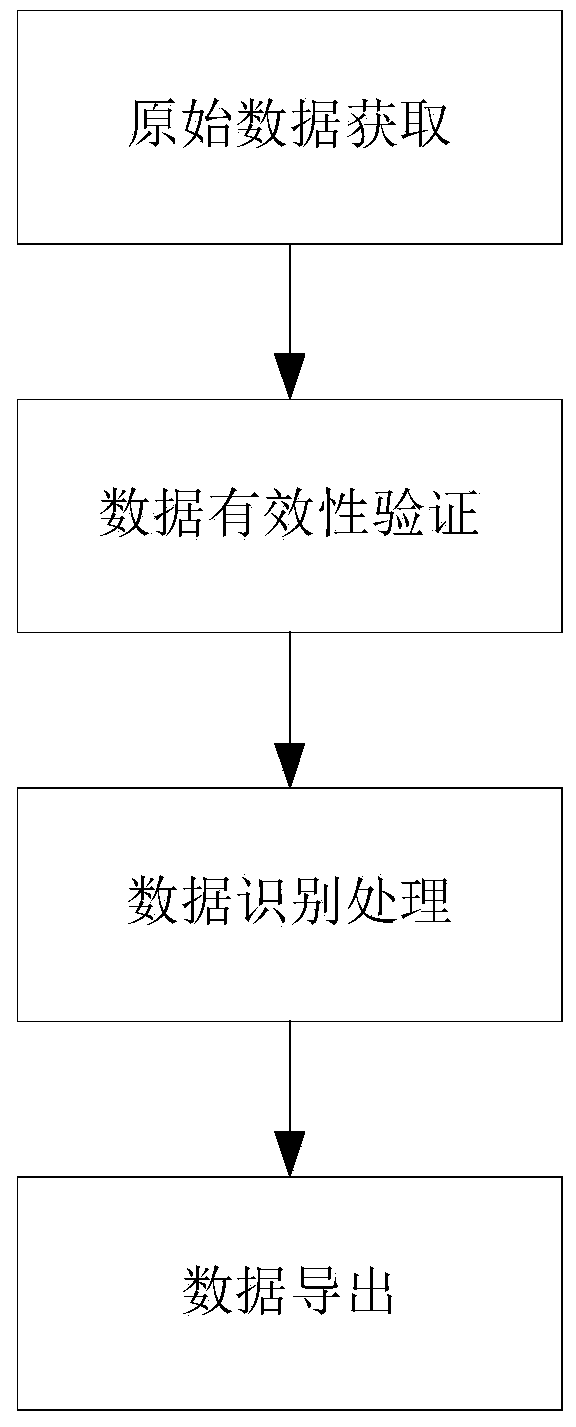
[0040] Such as figure 1 As shown, the visual recognition and screening method for drug trial adverse events disclosed by the present invention comprises the following steps:
[0041] Step 1. Obtain the baseline period data and test period data of each test subject from the EDC database;
[0042] Step 2, verify the validity of the obtained baseline data and test data, and select the test subjects with complete and valid baseline data and test data as test subjects to be identified;
[0043] Step 3, identify and process the baseline period data and test period data of each subject to be identified, and output visual data for easy identification;
[0044] In step 4, the visual data is popped up to facilitate visual review and verification.
[0045] By verifying the validity of the obtained baseline data and test data, it is possible to screen out complete and valid test subjects with baseline data and test data, thereby eliminating manual integrity and validity checks and effecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com