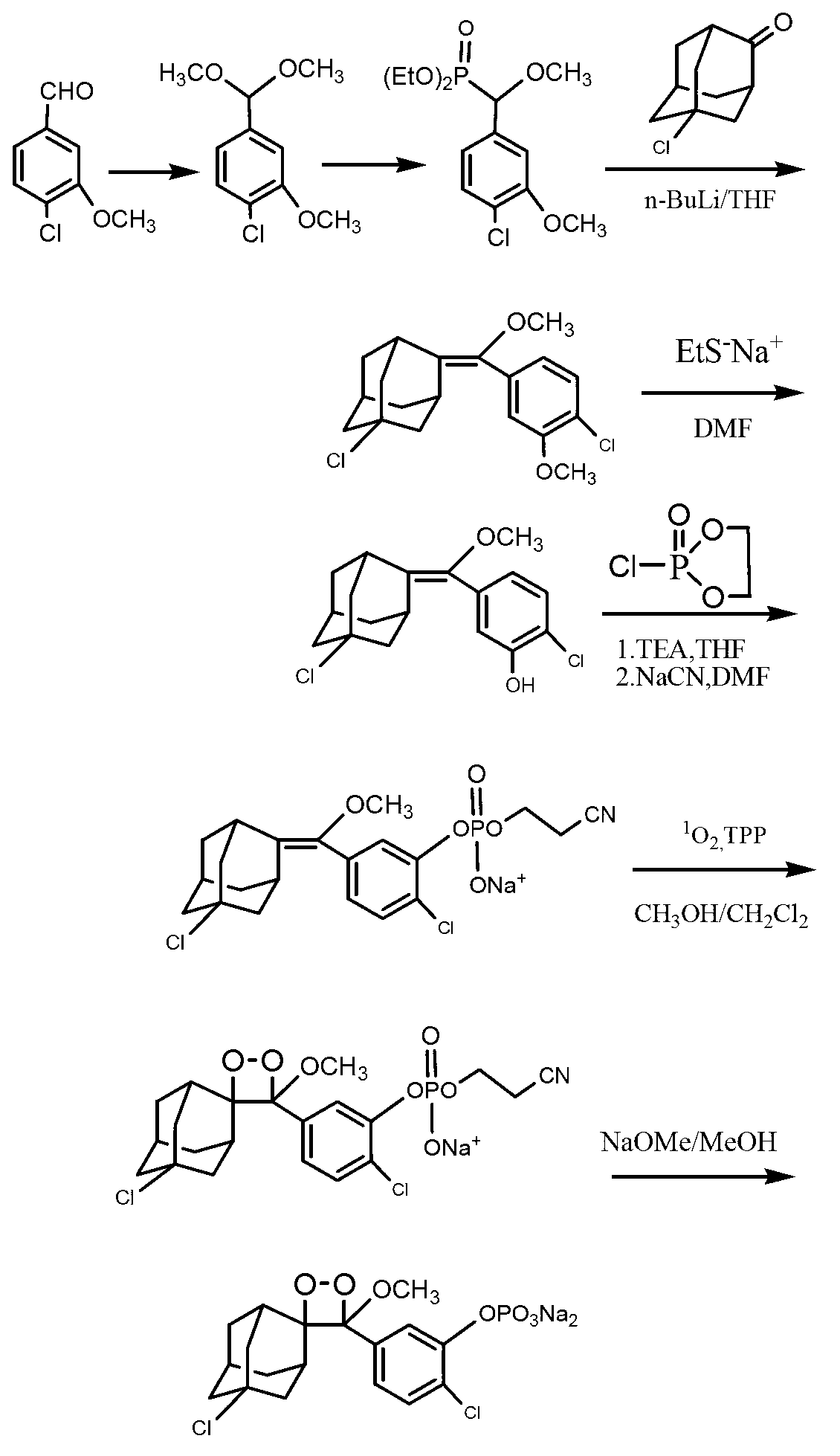
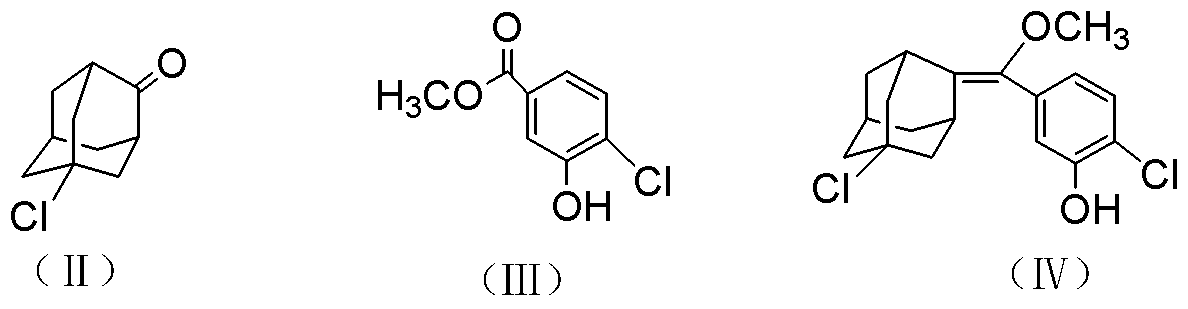
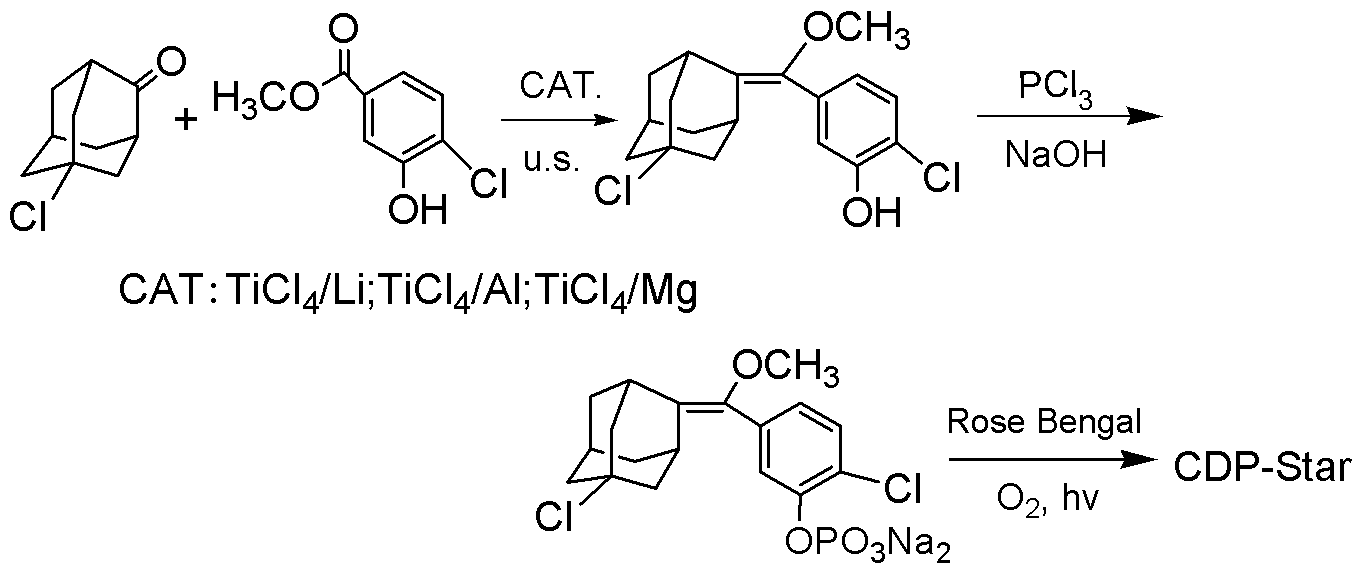
Synthetic method of 1, 2-dioxetane compound
A technology of dioxetane and a synthesis method, applied in the field of chemiluminescence substrate synthesis, can solve problems such as difficulty in realizing large-scale production, difficulty in separation and purification, complicated process, etc., achieve easy process amplification and industrialization, and improve reaction Yield, effect of improving reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of [(3-hydroxyl-4-chlorophenyl)methoxymethylene]-5-chloroadamantane: TiCl 4 / Li catalyst system
[0028] At room temperature (25°C), add 40mL of ethyl acetate to a 100mL three-necked flask, blow in nitrogen, and add 4.4mL of anhydrous TiCl 4 (40mmol), stir, obtain pale yellow liquid, add 0.56g (80mmol) metallic lithium, put three-necked bottle in ultrasonic cleaner, make the liquid level in the reaction flask slightly lower than the liquid level of water in the cleaning tank. Turn on the ultrasonic cleaner, and conduct an ultrasonic reaction at 25KHz for 15 minutes to obtain a black-purple liquid. Take out the three-necked bottle from the ultrasonic cleaner, add 15ml of triethylamine, and slowly add 5-chloro-2-adamantanone (20mmol) and 4-chloro-3 -Methyl hydroxybenzoate (20mmol) mixed solution, continue to reflux for 1h after adding, cool to room temperature, extract the reaction mixture three times with saturated sodium bicarbonate solution, an...
Embodiment 2
[0030] Example 2: Preparation of [(3-hydroxyl-4-chlorophenyl)methoxymethylene]-5-chloroadamantane: TiCl 4 / Al catalyst system
[0031] At room temperature, add 40mL tetrahydrofuran to a 100mL three-necked flask, blow nitrogen into it, and add 4.4mL anhydrous TiCl 4 (40mmol), stir, obtain pale yellow liquid, add 2.16g (80mmol) Al powder, put three-neck bottle in the ultrasonic cleaner, make the liquid level in the reaction bottle slightly lower than the liquid level of the water in the cleaning tank. Turn on the ultrasonic cleaner and react at 25KHz for 15min to obtain a black-purple liquid. Take out the three-neck bottle from the ultrasonic cleaner, add 15ml of triethylamine, and slowly add 5-chloro-2-adamantanone (20mmol) and 4-chloro-3-hydroxyl dissolved in 20mL of tetrahydrofuran dropwise under reflux Methyl benzoate (30mmol), continued to reflux for 2h after the addition, cooled the reaction solution to room temperature, extracted three times with ethyl acetate, combined...
Embodiment 3
[0033] Example 3: Preparation of [(3-hydroxyl-4-chlorophenyl)methoxymethylene]-5-chloroadamantane: TiCl 4 / Mg catalytic system
[0034] At room temperature, add 40mL ethyl acetate to a 100mL three-necked flask, blow nitrogen, and add 4.4mL anhydrous TiCl 4 (40mmol), stir, obtain light yellow liquid, add 1.92g (80mmol) Mg powder, put three-necked bottle in the ultrasonic cleaner, make the liquid level in the reaction bottle slightly lower than the liquid level of the water in the cleaning tank. Turn on the ultrasonic cleaner and react at 40KHz for 15min to obtain a black-purple liquid. Take out the three-necked bottle from the ultrasonic cleaner, add 15ml of triethylamine, and slowly add 5-chloro-2-adamantanone (20mmol) and 4-chloro-3 -Methyl hydroxybenzoate (40mmol), continue to reflux for 2h after addition, after cooling the reaction mixture to room temperature, extract three times with saturated aqueous sodium bicarbonate solution, combine the organic layers and wash with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com