Synthetic method of 1,2-dioxetane compound
A technology for the synthesis of dioxetane and its synthesis method, which is applied in the field of synthesis of chemiluminescent agents, can solve problems such as difficulty in large-scale production, long synthesis route, complicated operation, etc., and achieve fewer steps, short synthesis route and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
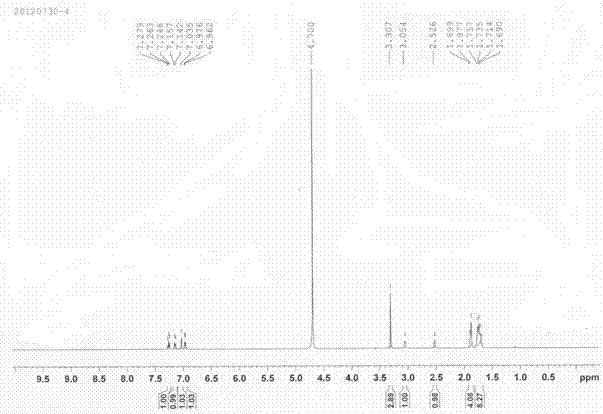
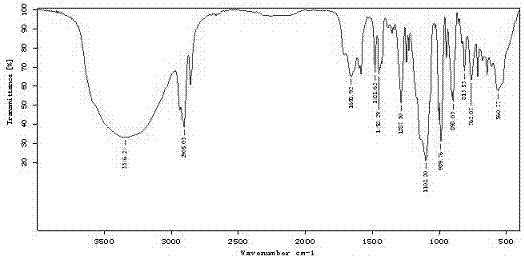
Image
Examples
Embodiment 1
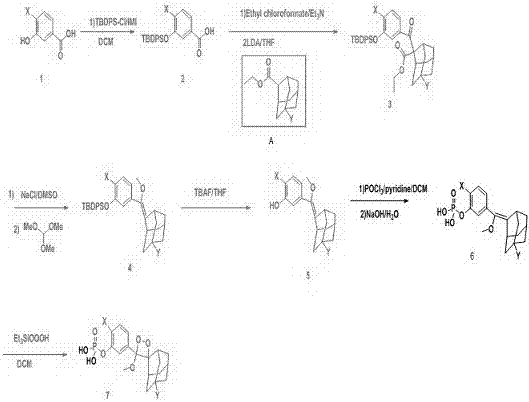
[0023] Embodiment one Synthesis of 3-(2′-spiroadamantane)-4-methoxy-4-(3″-phosphoryloxy)benzene-1,2-dioxetane, AMPPD.
[0024] The first step: the preparation of carboxylic acid compound II. 13.81g (100mmol) m-hydroxybenzoic acid, namely Dissolve in 300mL dichloromethane, cool to 0°C, add 54.97g (200mmol) tert-butyldiphenylchlorosilane and 13.62g (200mmol) imidazole successively, stir magnetically, and react at room temperature for 10h. Add 100mL saturated ammonium chloride solution after the monitoring reaction finishes, separate liquids and get the organic phase, this organic phase and the aqueous phase are extracted with 300mL ethyl acetate, and the organic phase obtained by liquid separation is combined, and the solvent is removed by distillation under reduced pressure with a rotary evaporator. Purify the product through the column to obtain 34.64g (92mmo) yellow oil, which is carboxylic acid compound II, namely .
[0025] The second step: the preparation of ester c...
Embodiment 2
[0032] Embodiment two 3-(2′-(5′-chloro)spiroadamantane)-4-methoxy-4-(3″-phosphoryloxy-4″-chloro)benzene-1,2-dioxetane Alkane, that is, the synthesis of CDP-Star.
[0033] The first step: the preparation of carboxylic acid compound II. 17.26 g (100 mmol) of 3-hydroxy-4-chlorobenzoic acid, namely Dissolve in 300mL of dichloromethane, cool to 0°C, add 54.97g (200mmol) tert-butyldiphenylchlorosilane and 13.62g (200mmol) imidazole, stir magnetically, and react at room temperature for 20h. Add 100mL saturated ammonium chloride solution after the monitoring reaction finishes, separate liquids and get the organic phase, this organic phase and the aqueous phase are extracted with 300mL ethyl acetate, and the organic phase obtained by liquid separation is combined, and the solvent is removed by distillation under reduced pressure with a rotary evaporator. Cross column purification product, obtain 38.63g (94mmol) carboxylic acid compound II, namely .
[0034] The second step: the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com