Preparation method of 1, 2-dioxetane derivative
A technology for dioxetane and derivatives is applied in the field of preparation of 1,2-dioxetane derivatives, which can solve the problems of being unsuitable for industrial production, difficult for industrial application, unfavorable for industrial production, and the like, and achieves a high yield High, convenient for industrial production, production safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
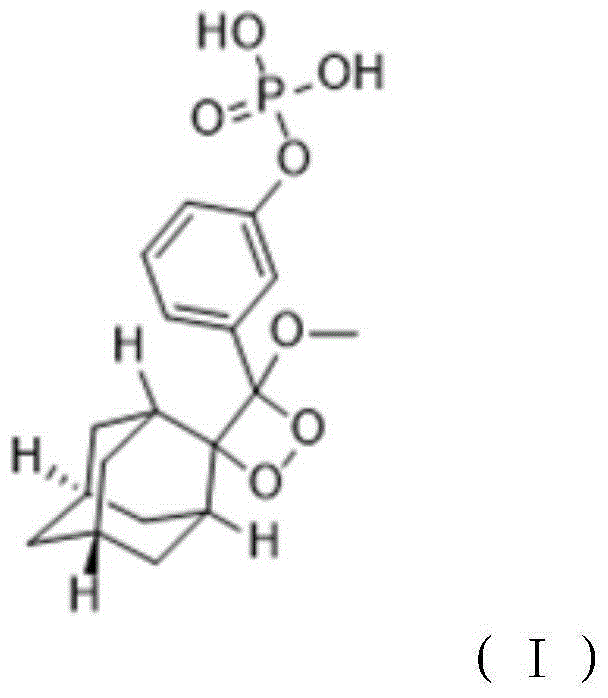
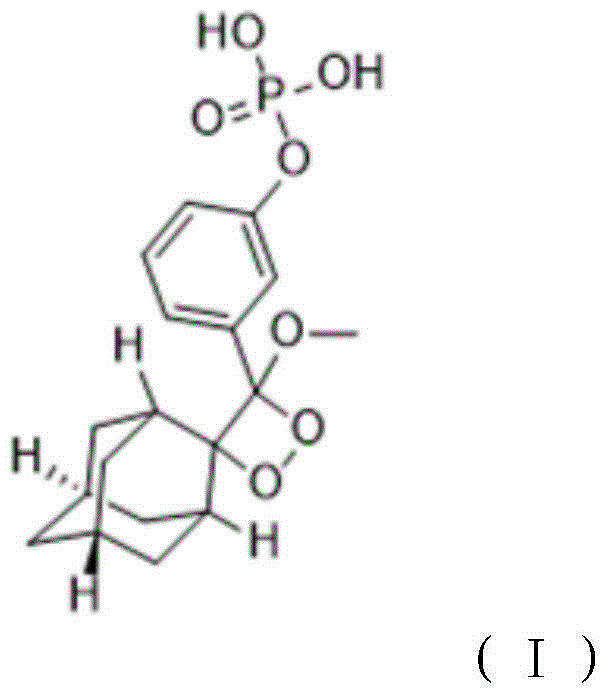
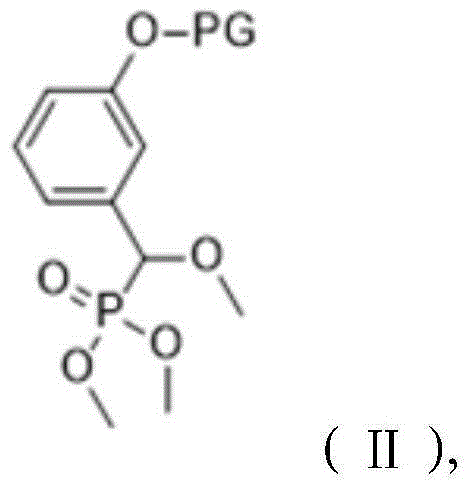
[0026] A method for preparing a 1,2-dioxetane derivative, the 1,2-dioxetane derivative is a compound shown in formula I, and the preparation method comprises the following steps: taking the compound shown in formula II 198g (0.5mol) of the compound is dissolved in 1000mL of methyl tert-butyl ether to obtain a solution of the compound shown in formula II with a molar volume concentration of 0.5mol / L; then at a temperature of -40°C, add 0.5mol The mixture of sodium hydride and 0.5mol adamantanone was then warmed up to room temperature and reacted for 10 hours; adding hydrochloric acid to adjust the pH value to 7.1, separating the organic layer, and then removing methyl tert-butyl ether by rotary evaporation to obtain the reactant; The reactant was dissolved in methanol, then the first sodium hydroxide solution was added, and the reaction was carried out for 2 hours. The molar ratio of the first sodium hydroxide to the compound shown in formula II was 1:1; then hydrochloric acid w...
Embodiment 2
[0031]A method for preparing 1,2-dioxetane derivatives. The substances of formula I, formula II, formula III and formula IV in this example are all the same as those in Example 1. The 1,2-dioxetane The cyclohexane derivative is a compound shown in formula I, and its preparation method comprises the steps of: taking the compound shown in formula II and dissolving it in an organic solvent to obtain the compound shown in formula II with a molar volume concentration of 0.4mol / L Then at a temperature of -20°C, add the mixture of alkali metal salt and adamantanone, then warm up to room temperature, and react for 10 hours, the moles of the compound shown in the formula II, alkali metal salt, and adamantanone The ratio is 1:0.9:0.9; add hydrochloric acid to adjust the pH value to 6.8, separate the organic layer, and then remove the organic solvent by rotary evaporation to obtain the reactant; dissolve the reactant with methanol, then add the first sodium hydroxide solution, and react ...
Embodiment 3
[0033] A preparation method of 1,2-dioxetane derivatives, the 1,2-dioxetane derivatives are compounds shown in formula I, formula I, formula II, formula The substances of III and Formula IV are all the same as in Example 1, and their preparation method comprises the steps of: taking the compound shown in Formula II and dissolving it in an organic solvent to obtain the compound shown in Formula II with a molar volume concentration of 0.6mol / L solution; then at a temperature of 20°C, add a mixture of alkali metal salt and adamantanone, then warm up to room temperature, and react for 12 hours. The molar ratio of the compound, alkali metal salt, and adamantanone shown in the formula II 1:1.1:1.1; add hydrochloric acid to adjust the pH value to 7.5, separate the organic layer, and then remove the organic solvent by rotary evaporation to obtain the reactant; dissolve the reactant with methanol, then add the first sodium hydroxide solution, and react 2.5 hours, the molar ratio of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com